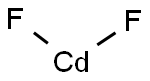
Cadmiumfluorid
|
|
Cadmiumfluorid Eigenschaften
- Schmelzpunkt:
- 1049 °C
- Siedepunkt:
- 1748 °C
- Dichte
- 6.33 g/mL at 25 °C(lit.)
- Flammpunkt:
- 1758°C
- L?slichkeit
- soluble in acid solutions; insoluble in ethanol
- Aggregatzustand
- Powder
- Wichte
- 6.64
- Farbe
- white
- Wasserl?slichkeit
- 4.3 g/100 mL (25 ºC)
- Crystal Structure
- CaF2 type
- crystal system
- Cube
- Merck
- 14,1619
- Solubility Product Constant (Ksp)
- pKsp: 2.19
- Space group
- Fm3m
- Lattice constant
a/nm b/nm c/nm α/o β/o γ/o V/nm3 0.539 0.539 0.539 90 90 90 0.1564
- CAS Datenbank
- 7790-79-6(CAS DataBase Reference)
- NIST chemische Informationen
- Cadmium difluoride(7790-79-6)
- EPA chemische Informationen
- Cadmium fluoride (CdF2) (7790-79-6)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | T+,N,T | ||
|---|---|---|---|
| R-S?tze: | 45-46-60-61-25-26-48/23/25-50/53 | ||
| S-S?tze: | 53-45-60-61 | ||
| RIDADR | UN 2570 6.1/PG 2 | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | EV0700000 | ||
| Hazard Note | Toxic | ||
| TSCA | Yes | ||
| HazardClass | 6.1 | ||
| PackingGroup | II | ||
| HS Code | 28261990 |
| Bildanzeige (GHS) |
  
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sicherheit |
|
Cadmiumfluorid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R45:Kann Krebs erzeugen.R46:Kann vererbbare Sch?den verursachen.
R60:Kann die Fortpflanzungsf?higkeit beeintr?chtigen.
R61:Kann das Kind im Mutterleib sch?digen.
R25:Giftig beim Verschlucken.
R26:Sehr giftig beim Einatmen.
R48/23/25:Giftig: Gefahr ernster Gesundheitssch?den bei l?ngerer Exposition durch Einatmen und durch Verschlucken.
R50/53:Sehr giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
S-S?tze Betriebsanweisung:
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S60:Dieses Produkt und sein Beh?lter sind als gef?hrlicher Abfall zu entsorgen.
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
Chemische Eigenschaften
White powderPhysikalische Eigenschaften
Colorless cubic crystal; density 6.33 g/cm3; melts at 1,110°C; vaporizes at 1,748°C; vapor pressure 5 torr at 1,231°C; moderately soluble in water, 4.35 g/100mL at 25°C; soluble in hydrofluoric and other mineral acids; practically insoluble in alcohol and liquid ammonia.Verwenden
Cadmium fluoride is used in phosphors, glass and nuclear reactor controls. It is also used in high-temperature lubricants and to start crystals for lasers. It is used in oxygen-sensitive applications such as the production of metallic alloys and it is also used in limited medical treatment protocols. In addition, it can be used in synthetic organic chemistry.Definition
Available as pure crystals, 99.89%, d 6.6, mp approx- imately 1110C, soluble in water and acids, insoluble in alkalies.synthetische
Cadmium fluoride is prepared by the reaction of gaseous fluorine or hydrogen fluoride with cadmium metal or its salt, such as chloride, oxide or sulfide:Cd + F2 → CdF2
Cd + 2HF → CdF2 + H2
CdO + 2HF → CdF2 + H2O
It also may be obtained by dissolving cadmium carbonate in 40% hydrofluoric acid solution, evaporating the solution and drying in vacuum at 150°C:
CdCO3 + 2HF → CdF2 + H2O + CO2
It also may be prepared by mixing cadmium chloride and ammonium fluoride solutions, followed by crystallization.
Sicherheitsprofil
Confirmed human carcinogen. Poison by subcutaneous route. Violent reaction with K. When heated to decomposition it emits very toxic fumes of Cd and F-. See also FLUORIDES and CADMIUM COMPOUNDS.l?uterung methode
Crystallise it by dissolving it in hot water (25mL/g at room temperature) at 60o, filtering, then cooling. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 243 1963.]Cadmiumfluorid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Cadmiumfluorid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 110)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29810 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 |
admin@zhanyaobio.com | China | 2123 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 8670 | 58 |
| GLR Innovations | +91 9891111994 |
info@glrgroup.in | India | 4535 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18148 | 58 |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 |
sales@senfeida.com | China | 23046 | 58 |
7790-79-6(Cadmiumfluorid)Verwandte Suche:
Ammoniumfluorid
FLUORIDE STANDARD
Cadmium
Fluorwasserstoff
Natriumfluorid
Aluminiumfluorid
Ammoniumhydrogendifluorid
Kaliumfluorid
Calciumsulfid
Cobaltdithiocyanat
Cadmiumhydroxid
Beryllium
Kaliumchromat
Schwefelhexafluorid
Bortrifluorid
sodium fluoride
Cadmiumfluorid
- Cadmium fluoride (CdF2)
- Cadmium fluorure
- cadmiumfluoride(cdf2)
- cadmiumfluorure
- Cadmium Flouride
- Cadmium fluoride 1g [7790-79-6]
- Cadmium fluoride≥98%
- CADMIUM FLUORIDE 99.98%
- CADMIUM FLUORIDE (99.98%-CD)
- Cadmiumfluoride,Puratronic?,99.99%(metalsbasis)
- Cadmiumfluoride,99%
- Cadmium fluoride, 99.99+%
- cadmium fluoride, puratronic
- Cadmium fluoride, Puratronic(R), 99.99% (metals basis)
- CdF2
- CADMIUM (II) FLUORIDE
- cadmium difluoride
- CADMIUM FLUORIDE
- Cadmium fluoride, 99.9% (metals basis)
- Cadmium fluoride 99.9%
- cadmium(2+) difluoride
- 7790-79-6
- 7790-76-9
- Cd2F
- Catalysis and Inorganic Chemistry
- Cadmium
- metal halide
- Cadmium Salts
- CadmiumMetal and Ceramic Science
- Catalysis and Inorganic Chemistry
- Chemical Synthesis
- Salts