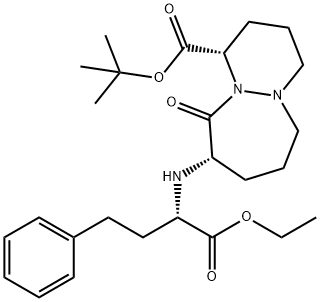
Cilazapril EP Impurity A
|
|
Cilazapril EP Impurity A Eigenschaften
- Siedepunkt:
- 580.4±60.0 °C(Predicted)
- Dichte
- 1.17±0.1 g/cm3(Predicted)
- pka
- 5.26±0.40(Predicted)
Sicherheit
Cilazapril EP Impurity A Chemische Eigenschaften,Einsatz,Produktion Methoden
Cilazapril EP Impurity A Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Cilazapril EP Impurity A Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 5)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| QUALITY CONTROL SOLUTIONS LTD. | 0755-66853366; 13670046396 |
ORDERS@QCSRM.COM | China | 24342 | 58 |
| Suzhou Haiben Pharmaceutical Co., Ltd | 14760821013 13564957716 |
1816280386@qq.com | China | 8045 | 58 |
| guoyungurui | 18162595016; 18162595016 |
3287908757@qq.com | China | 10335 | 58 |
| Henan Vcommend Consultrading Co., Ltd. | 13393721940; 13393721940 |
sales@vcommend.com | China | 10005 | 58 |
- Cilazapril EP Impurity A
- 6H-Pyridazino[1,2-a][1,2]diazepine-1-carboxylic acid, 9-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]octahydro-10-oxo-, 1,1-dimethylethyl ester, (1S,9S)-
- 106860-19-9