2-ACETYL-4-CHLOROTHIOPHENE
|
|
|
- CAS-Nr.
- 34730-20-6
- Englisch Name:
- 2-ACETYL-4-CHLOROTHIOPHENE
- Synonyma:
- 1-(4-chlorothiophen-2-yl)ethan-1-one;Avatrombopag Impurity 26;4-chloro-2-acetothiophene;Avatroposide impurities33;2-ACETYL-4-CHLOROTHIOPHENE;2-acetyl-4-chlopothiophene;2-Acetyl-4-chlorothiophene, 98;1-(4-chlorothien-2-yl)ethanone;Ethanone, 1-(4-chloro-2-thienyl)-;1-(4-Chlorothiophen-2-yl)ethanone
- CBNumber:
- CB9690448
- Summenformel:
- C6H5ClOS
- Molgewicht:
- 160.62
- MOL-Datei:
- 34730-20-6.mol
|
2-ACETYL-4-CHLOROTHIOPHENE Eigenschaften
- Siedepunkt:
- 263.0±25.0 °C(Predicted)
- Dichte
- 1.334
- storage temp.
- Keep in dark place,Sealed in dry,Room Temperature
- InChI
- InChI=1S/C6H5ClOS/c1-4(8)6-2-5(7)3-9-6/h2-3H,1H3
- InChIKey
- FKESGQASARHBDC-UHFFFAOYSA-N
- SMILES
- C(=O)(C1SC=C(Cl)C=1)C
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| R-S?tze: |
36 |
|
|
| S-S?tze: |
26 |
|
|
| RIDADR |
UN2810 |
|
|
| HazardClass |
6.1 |
|
|
| HS Code |
2934999090 |
|
|
| Bildanzeige (GHS) |

|
| Alarmwort |
Warnung |
| Gefahrenhinweise |
| Code |
Gefahrenhinweise |
Gefahrenklasse |
Abteilung |
Alarmwort |
Symbol |
P-Code |
| H302 |
Gesundheitssch?dlich bei Verschlucken. |
Akute Toxizit?t oral |
Kategorie 4 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P270, P301+P312, P330, P501 |
| H312 |
Gesundheitssch?dlich bei Hautkontakt. |
Akute Toxizit?t dermal |
Kategorie 4 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P280,P302+P352, P312, P322, P363,P501 |
| H331 |
Giftig bei Einatmen. |
Akute Toxizit?t inhalativ |
Kategorie 3 |
Achtung |
![GHS hazard pictograms]() src="/GHS06.jpg" width="20" height="20" /> src="/GHS06.jpg" width="20" height="20" /> |
P261, P271, P304+P340, P311, P321,P403+P233, P405, P501 |
|
| Sicherheit |
| P261 |
Einatmen von Staub vermeiden. |
| P304+P340 |
BEI EINATMEN: Die Person an die frische Luft bringen und für ungehinderte Atmung sorgen. |
| P405 |
Unter Verschluss aufbewahren. |
|
2-ACETYL-4-CHLOROTHIOPHENE Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
2-Acetyl-4-chlorothiophene is an oxychloride that belongs to the family of thiourea derivatives. It is synthesized by reacting phosphorus oxychloride with 2,3-dichloroacetophenone in a solvent such as dioxane or acetonitrile. The final product is purified by means of vacuum distillation and recrystallization from diethyl ether, hexane, and chlorinated hydrocarbons.
Reaktionen
Pd(OAc)2 catalysed the direct arylation of some functionalized halothiophene derivatives allowing the synthesis in only one step of polyfunctionalized arylated thiophenes. In the presence of 2-acetyl-4-chlorothiophene and various aryl bromides, the 5-arylation products were obtained in moderate to high yields employing only 0.5 mol% catalyst. Researchers studied the coupling of 2-acetyl-4-chlorothiophene with several aryl bromides employing 0.5 mol% Pd(OAc)2 as the catalyst and KOAc as the base. These phosphine-free catalyst reaction conditions allowed the successful coupling of several aryl bromides to more simple thiophene derivatives. Using such conditions, the 5-arylated thiophenes were obtained with high isolated yields. With this procedure, the priority for the arylation of this 2,4-disubstituted thiophene is the 5-position. Moreover, no formation of by-products, such as thiophene oligomers, due to the oxidative addition of this chlorothiophene to palladium was detected during these reactions[1].
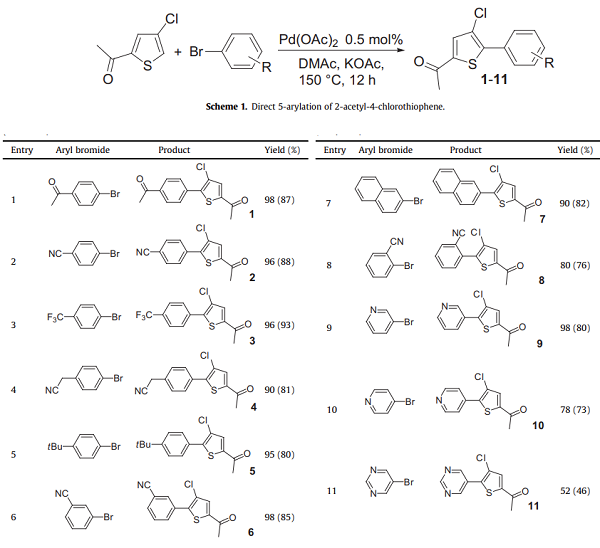
2-ACETYL-4-CHLOROTHIOPHENE Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
2-ACETYL-4-CHLOROTHIOPHENE Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 190)Lieferanten
34730-20-6()Verwandte Suche:
- 2-ACETYL-4-CHLOROTHIOPHENE
- 1-(4-chlorothien-2-yl)ethanone
- 2-Acetyl-4-chlorothiophene, 98
- 1-(4-Chlorothiophen-2-yl)ethanone
- Ethanone, 1-(4-chloro-2-thienyl)-
- 2-acetyl-4-chlopothiophene
- 4-chloro-2-acetothiophene
- Avatrombopag Impurity 26
- 2-ACETYL-4-CHLOROTHIOPHENE ISO 9001:2015 REACH
- 1-(4-chlorothiophen-2-yl)ethan-1-one
- Avatroposide impurities33
- 1-(3,4-dichloro-6-((4-(4-chlorothiophen-2-yl)-5-(4-cyclohexylpiperazin-1-yl)thiazol-2-yl)carbamoyl)pyridin-2-yl)piperidine-4-carboxylic acid
- 34730-20-6
- Sulfur compounds
- Intermediate

