Deuterium Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R12:Hochentzündlich.
S-S?tze Betriebsanweisung:
S9:Beh?lter an einem gut gelüfteten Ort aufbewahren.
S16:Von Zündquellen fernhalten - Nicht rauchen.
S33:Ma?nahmen gegen elektrostatische Aufladungen treffen.
Chemische Eigenschaften
colourless gas
Verwenden
Used extensively in small amounts as tracer in the establishment of rates and kinetics of chemical reactions.
Definition
A naturally occurring stable isotope of
hydrogen in which the nucleus contains
one proton and one neutron. The atomic
mass is thus approximately twice that of
1H; deuterium is known as ‘heavy hydrogen’.
Chemically it behaves almost identically
to hydrogen, forming analogous
compounds, although reactions of deuterium
compounds are often slower than
those of the corresponding 1H compounds.
This is made use of in kinetic studies where
the rate of a reaction may depend on transfer
of a hydrogen atom (i.e. a kinetic isotope
effect).
Allgemeine Beschreibung
DEUTERIUM is an isotope of hydrogen but DEUTERIUM is chemically identical. DEUTERIUM is a colorless, odorless gas. DEUTERIUM is easily ignited. Once ignited DEUTERIUM burns with a pale blue, almost invisible flame. The vapors are lighter than air. DEUTERIUM is flammable over a wide range of vapor/air concentrations. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. DEUTERIUM is not toxic but is a simple asphyxiate by the displacement of oxygen in the air.
Air & Water Reaktionen
Highly flammable.
Reaktivit?t anzeigen
DEUTERIUM, like hydrogen, is a reducing agent; reacts readily with oxidizing agents.
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Some may be irritating if inhaled at high concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases.
Brandgefahr
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), DEUTERIUM (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and DEUTERIUM fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Landwirtschaftliche Anwendung
Deuterium is one of the three isotopes of hydrogen, the other two being hydrogen-1 and tritium. Hydrogen-1 and deuterium are naturally occurring stable isotopes, while the radioactive tritium is made artificially.
In nature, the ratio is one part of deuterium to 6500parts of normal hydrogen. Deuterium is present in water as the oxide HDO from which deuterium is usually obtained by electrolysis or fractional distillation. Its chemical behavior is similar to that of hydrogen, although deuterium compounds react slowly.
Synthese
Deuterium can be made by reacting D2O with metallic elements (Na, Mg and U). The reaction equation is shown below:
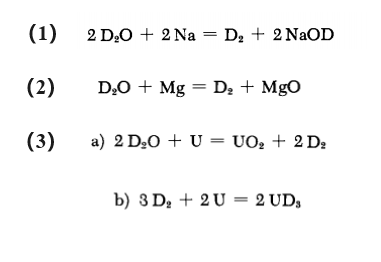
l?uterung methode
Pass the gas over activated charcoal at -195o [MacIver & Tobin J Phys Chem 64 451 1960]. Purify it also by diffusion through nickel [Pratt & Rogers, J Chem Soc, Faraday Trans I 92 1589 1976]. Always check deuterium for radioactivity to determine the amount of tritium in it (see D2O below).
Deuterium Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

