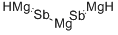Antimon, Verbindung mit Magnesium (2:3) Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Magnesium antimonide has been prepared by the
reaction of Mg and Sb metals. Mg melts at 652°C
and is stable in an inert atmosphere until its boiling
point of 1091°C is reached. Since Sb melts at 630.6°C
and is stable in an inert atmosphere until its boiling
point of 1587°C, the reaction is best carried out by
evaporating Mg metal at about 680°C onto molten
Sb metal.
Chemische Eigenschaften
hexagonal; 6mm pieces and smaller with 99.5% purity [CER91] [CRC10]
Physikalische Eigenschaften
Its molecular weight is 316.4351 g/mol and its CAS
number is 12057-75-9. Mg
3Sb
2 melts at 1245°C. Structural
stabilities elastic properties and electronic structures
have been determined from first-principles
calculation by using Castep and Dmol computer
programs based on the density functional theory given
for the Mg
3Sb
2 phase.
Verwenden
Magnesium antimonide has also been used in
an electrochemical and device apparatus for high-amperage
electrical energy storage. It features a hightemperature,
all-liquid chemistry. The batteries are
simply tanks filled with three separate layers of liquid
that float on top of one another at 700°C: the top layer
is molten magnesium, the bottom is antimony, and the
middle layer is a salt containing magnesium antimonide,
a dissolved compound of the two metals.
synthetische
Magnesium antimonide has been prepared by the
reaction of Mg and Sb metals. Mg melts at 652°C
and is stable in an inert atmosphere until its boiling
point of 1091°C is reached. Since Sb melts at 630.6°C
and is stable in an inert atmosphere until its boiling
point of 1587°C, the reaction is best carried out by
evaporating Mg metal at about 680°C onto molten
Sb metal.
Antimon, Verbindung mit Magnesium (2:3) Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte