(S)-2-Fluor-α-methyl[1,1'-biphenyl]-4-essigsure Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R25:Giftig beim Verschlucken.
S-S?tze Betriebsanweisung:
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
Beschreibung
(S)-Flurbiprofen is the COX-active enantiomer of the non-selective COX inhibitor flurbiprofen with IC
50 values of 0.48 and 0.47 μM for COX-1 and COX-2, respectively, in guinea pig whole blood. It inhibits the release of 6-keto prostaglandin F
1α (6-keto-PGF
1α; ) and thromboxane B
2 (TXB
2; ) from rat whole blood, gastric mucosa, lung, and jejunal tissue
ex vivo in a dose-dependent manner. (S)-Flurbiprofen (1 nM) inhibits basal and bradykinin-, serotonin-, and histamine-stimulated prostaglandin E
2 (PGE
2) release from isolated skin flaps of rat lower hind paws. It also inhibits release of the neuroinflammatory marker calcitonin gene-related peptide (CGRP; Item Nos.
24405 |
24725 |
24728) when used at a concentration of 1 μM.
In vivo, (S)-flurbiprofen reduces the number of flinches per minute in the formalin test in rats, indicating antinociceptive activity.
Verwenden
(S)-Flurbiprofen is the S-isomer of Flurbiprofen (F598700), an anti-inflammatory used as an analgesic.
Synthese
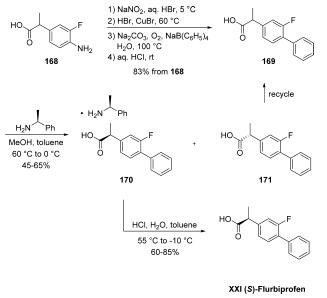
The synthesis began with conversion of commercially
available aniline 168 to racemic flurbiprofen (169) through a Sandmeyer reaction and subsequent phenyl
group introduction through the use of sodium tetraphenylborate.
A chiral resolution was then performed on the resulting
stereogenic acid on multikilogram scale by treatment with (S)-
1-phenylethylamine in MeOH/toluene, which gave various
yields of salt 170 as reported by the authors. Acidification
with aqueous HCl delivered (S)-flurbiprofen (XXI). Importantly,
with respect to green chemistry considerations, the (R)-
enantiomer could be recycled by racemizing the undesired (R)-
enantiomer 171 in refluxing methanolic sulfuric acid, improving
the overall atom economy of the process and significantly
reducing waste.
(S)-2-Fluor-α-methyl[1,1'-biphenyl]-4-essigsure Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

