2-Methylpropylisocyanid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R11:Leichtentzündlich.
R23:Giftig beim Einatmen.
S-S?tze Betriebsanweisung:
S16:Von Zündquellen fernhalten - Nicht rauchen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
Beschreibung
tert-Butyl isocyanide is a key regent for synthesizing many heterocycles and can undergo many cyclization reactions1. It is a colorless liquid, with a strong smell, bench-stable, and soluble in most organic solvents, including methanol, ethanol, ether, toluene, and dichloromethane. Many combinations of 5-member heterocycles with C, N, S, and O atoms, and even boron-containing cycles can be done via [4+1] cycloaddition. Except for heterocyclization, the reagent can be used as a mild esterification reagent, especially in the chemistry of β-lactam antibiotics, penicillins, and cephalosporins. The Passerini reaction with α-geminal polyhalogenated aldehydes allows easy esterification of such intricate substrates.
Chemische Eigenschaften
clear colorless liquid
Verwenden
tert-Butyl isocyanide is used in a tin-free procedure for alkyl radical reactions in the presence of a free-radical initiator. It is a useful intermediate in multicomponent reactions. It is also used in the synthesis of coumarines, 4H-chromenes, isoxazolines and to trap 2-cyclopropylidene-1,3-diones.
synthetische
CAUTION: Use a well-ventilated hood and observe all precautions when using hydrogen cyanide.
To a 1-1, stainless steel Hoke pressure cylinder in a well-ventilated hood is added 196 gm (3.5 moles) of isobutene, 95 gm (3.5 moles) of hydrogen cyanide, and 100 gm (0.7 mole) or cuprous bromide. The cylinder is closed, shaken for 15 hr at 70°C, cooled to room temperature, and vented cautiously in the hood, and the viscous residue is poured slowly into aqueous potassium cyanide (260 gm in 100 ml of water) to decompose the cuprous complex, liberating an organic layer. The organic layer is separated, dried, and distilled under reduced pressure to afford 97 g tert-Butyl isocyanide, b.p. 60-63°C (314 mm Hg), ng 1.3749, IR 2143 s, 1472 m, 1368 m, 1230 m, 1208 m, 855 w c m - 1; yield, 33% based on starting isobutylene; 16% based on CuBr.
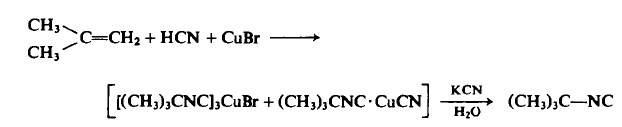
Allgemeine Beschreibung
Carbon dative bond formation by
tert-butyl isocyanide on the germanium (100) surface has been reported. It also forms complexes with metals and inserts into metal-carbon bonds to form iminoacyls.
2-Methylpropylisocyanid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

