Mobocertinib Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Mobocertinib is derived from osimertinib, a
second-generation TKI which exhibits only limited
activity against several resistant EGFRex20ins
mutants. Both inhibitors are structurally identical
except that the pyrimidine ring of moboceritinib
incorporates a snugly fitting isopropyl ester group that targets a previously unoccupied pocket,
resulting in expanded coverage of EGFRex20ins
mutations as well as improved selectivity over wildtype EGFR vs. osimertinib.
Verwenden
Mobocertinib (TAK-788) is an orally active and irreversible EGFR/HER2 inhibitor. Mobocertinib effectively inhibits oncogenic variants containing mutations that activate the EGFRex20ins, with selectivity superior to that of wild-type EGFR. Mobocertinib is FDA-approved for the treatment of patients with non-small cell lung cancer (NSCLC) who have a HER2 mutation or an EGFR mutation, including an exon 20 insertion mutation. Mobocertinib is approved by the FDA for the treatment of patients with non-small cell lung cancer (NSCLC) who have HER2 mutations or EGFR mutations, including exon 20 insertion mutations.
Trademarks
Exkivity
TM
Stoffwechsel
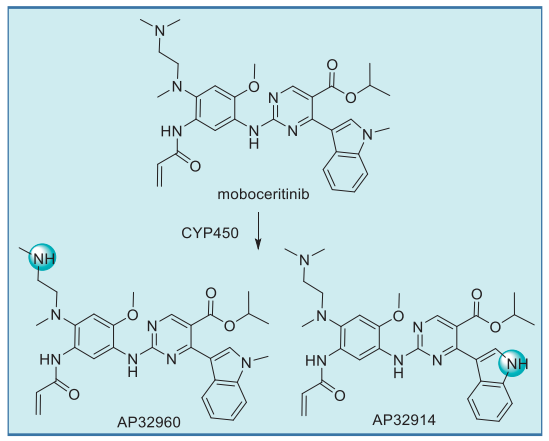
Moboceritinib undergoes CYP450-mediated metabolism to give two primary N-demethylated metabolites, AP32914 and AP32960, whose IC50 values are within 2-fold of mobocertinib for both wt and mutant EGFR. It is likely that these metabolites also contribute to the pharmacologic activity of mobocertinib. Interestingly, the typically labile isopropyl ester appears resistant to esterase-induced hydrolysis.
Mobocertinib Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

