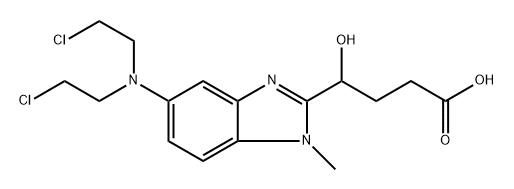
Bendamustine Impurity 5
|
|
Bendamustine Impurity 5 Eigenschaften
- Siedepunkt:
- 624.5±55.0 °C(Predicted)
- Dichte
- 1.39±0.1 g/cm3(Predicted)
- pka
- 4.33±0.10(Predicted)
Sicherheit
Bendamustine Impurity 5 Chemische Eigenschaften,Einsatz,Produktion Methoden
Bendamustine Impurity 5 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Bendamustine Impurity 5 Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 8)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 |
overseasales@yongstandards.com | China | 11922 | 58 |
| Cato Research Chemicals Inc. | 020-81215950 4000-868-328 |
customer@uwalab.com | United States | 10404 | 58 |
| Shenzhen Roark Pharma-Tec Co., Ltd. | 13128912434 |
2880126944@qq.com | China | 2577 | 58 |
| Shanghai Zerui Biotechnology Co., Ltd. | 021-54580889 18321124657 |
731997554@qq.com | China | 10608 | 58 |
| Hubei De'ao Chemical Research Pharmaceutical Technology Co., Ltd | 18502726233; 18502726233 |
3641133981@qq.com | China | 9713 | 58 |
- Bendamustine Impurity 5
- Hydroxybendamustin sodium salt
- 5-[Bis(2-chloroethyl)amino]-γ-hydroxy-1-methyl-1H-benzimidazole-2-butanoic Acid
- 1H-Benzimidazole-2-butanoic acid, 5-[bis(2-chloroethyl)amino]-γ-hydroxy-1-methyl-
- 1138238-08-0