2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride
|
|
|
- CAS-Nr.
- 42036-65-7
- Englisch Name:
- 2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride
- Synonyma:
- NSC 12467;NSC 620461;Tramadol EP Impurity E;Tramadol Impurity E HCl;Tramadol EP Impurity E HCl;TraMadol Related CoMpound B;TraMadol Hydrochloride IMpurity-E(EP);Tramadol stg-I mannich HCL EP imp-E RC-B;2-(DIMETHYLAMINOMETHYL) CYCLOHEXANONE HCL;2-(Dimethylaminomethyl)-1-cyclohexanoneHCl
- CBNumber:
- CB7214624
- Summenformel:
- C9H18ClNO
- Molgewicht:
- 191.7
- MOL-Datei:
- 42036-65-7.mol
|
2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride Eigenschaften
- Schmelzpunkt:
- 147-151 °C
- storage temp.
- 2-8°C
- L?slichkeit
- Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly)
- Aggregatzustand
- Crystalline Powder
- Farbe
- White
- Wasserl?slichkeit
- soluble
- InChI
- InChI=1S/C9H17NO.ClH/c1-10(2)7-8-5-3-4-6-9(8)11;/h8H,3-7H2,1-2H3;1H
- InChIKey
- CLVHTSWMNNSUSH-UHFFFAOYSA-N
- SMILES
- C1(CN(C)C)CCCCC1=O.Cl
- CAS Datenbank
- 42036-65-7(CAS DataBase Reference)
2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride Chemische Eigenschaften,Einsatz,Produktion Methoden
S-S?tze Betriebsanweisung:
S24/25:Berührung mit den Augen und der Haut vermeiden.
Chemische Eigenschaften
white crystalline powder
Verwenden
An impurity found in Tramadol (T712500).
Synthese
0.982 g (1.03 mL, 10.0 mmol) cyclohexanone, 0.360 g (12.0 mmol) paraformaldehyde, 0.816 g (10.0 mmol) dimethylammonium chloride and 4 mL ethanol are filled in a 25 mL round bottom flask with reflux condenser and magnetic stir bar. 2 drops of conc. hydrochloric acid is added, and the mixture is heated under stirring for 4 hours under reflux. The hot solution is filtered in a round bottom flask, and the solvent is evaporated by the rotary evaporator. The residue is dissolved in 2 mL ethanol under heating. At room temperature, 20 mL acetone is added to the solution. For complete crystallization, the solution is stored overnight in the freezer compartment. The crystallized crude product 2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride is sucked off over a Buechner funnel and dried in the desiccator over silica gel.
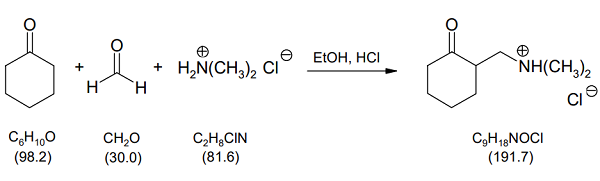
2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 169)Lieferanten
42036-65-7()Verwandte Suche:
- 2-(DIMETHYLAMINOMETHYL)-1-CYCLOHEXANONE HYDROCHLORIDE
- 2-(DIMETHYLAMINOMETHYL)-CYCLOHEXAN-1-ONE HCL
- 2-(DIMETHYLAMINOMETHYL) CYCLOHEXANONE HCL
- 2-[(DIMETHYLAMINO)METHYL]CYCLOHEXANONE HYDROCHLORIDE
- 2-(N,N-DIMETHYLAMINOMETHYL)CYCLOHEXANONE HCL
- 2-(Dimethylaminomethyl)-1-cyclohexanone, hydrochloride salt
- 2-(Dimethylamino)methyl cyclhexanone hydrochloride
- 2-(Dimethylaminomethyl)-1-cyclohexanoneHCl
- 2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride 98%
- 2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride,98%
- 2-Dimethyl Amino Methyl Cyclohexanone.HCL (Menich Base)
- Tramadol Related Compound B (25 mg) (2-(dimethylaminomethyl)-1-cyclohexanone hydrochloride)
- 2-(DiMethylaMinoMethyl)-1-cyclohexanone hydrochlor
- 2-(DiMethylaMinoMethyl)-1-cyclohexanone hydrochloride, 98% 1GR
- 2-(BisMethyl)aMinoMethylcyclohexanone Hydrochloride
- DiMethyl((2-oxocyclohexyl)Methyl)aMMoniuM Chloride
- NSC 12467
- NSC 620461
- Cyclohexanone, 2-[(diMethylaMino)Methyl]-, hydrochloride
- TraMadol Hydrochloride IMpurity-E(EP)
- TraMadol Related CoMpound B
- Tramadol EP Impurity E
- Tramadol Impurity 5(Tramadol EP Impurity E)
- 2-dimethylaminomethyl cyclohexanon hydrochloride
- Tramadol Impurity E HCl
- Tramadol EP Impurity E HCl
- 2 - DIMETHYL - METHYL - 1 CYCLOHEXANONE HYDROCHLORIDE
- Tramadol stg-I mannich HCL EP imp-E RC-B
- Tramadol EP Impurity E/ Tramadol Related Compound B
- Tramadol Related Compound B (2-(dimethylaminomethyl)-1-cyclohexanone hydrochloride) (1672621)
- Tramadol USP Related Compound B as Hydrochloride
- Tramadol Hydrochloride EP Impurity E as Hydrochloride
- Tramadol Related Compound B (25 mg) (2-[(Dimethylamino)methyl]cyclohexanone hydrochloride)
- 2-(Dimethylaminomethyl)-1-cyclohexanone hydrochloride/DMCH
- Tramadol EP Impurity E HCl (Tramadol USP Related Compound B)
- (2RS)-2-[(Dimethylamino)methyl]cyclohexanone Hydrochloride
- 42036-65-7
- C9H17NOClH
- C9H17NOHCl
- Aminomethyl's
- Ring Systems

