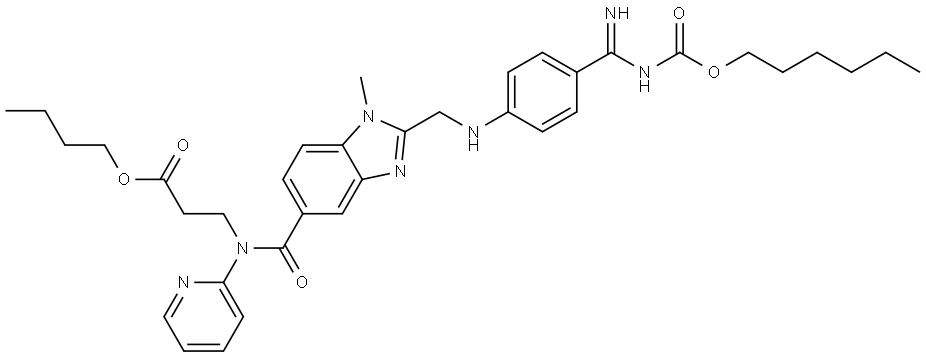
Dabigatran Impurity 36
|
|
Dabigatran Impurity 36 Eigenschaften
- Dichte
- 1.22±0.1 g/cm3(Temp: 20 °C; Press: 760 Torr)(Predicted)
- pka
- 9.88±0.46(Predicted)
Sicherheit
Dabigatran Impurity 36 Chemische Eigenschaften,Einsatz,Produktion Methoden
Dabigatran Impurity 36 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Dabigatran Impurity 36 Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 4)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Standardpharm Co. Ltd. | 86-714-3992388 |
overseasales1@yongstandards.com | United States | 14332 | 58 |
| Nanjing Huanuo Biomedical Technology Co., Ltd | 18913906212 |
2885426135@qq.com | China | 7160 | 58 |
| Guangzhou Zhenlin Pharmaceutical Technology Co., Ltd | 18102736234 18102736234 |
2208093734@qq.com | China | 7664 | 58 |
- β-Alanine, N-[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl]phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl-, butyl ester
- 1688681-89-1