SULPHIDE Chemische Eigenschaften,Einsatz,Produktion Methoden
synthetische
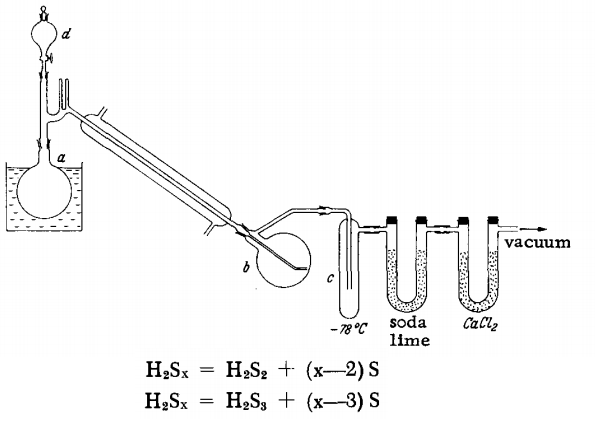
First, a (300 ml) flask is heated in a paraffin bath to 110°C while the apparatus is evacuated to 12-15 mm. Then 15 ml of HaSx is introduced through d. After a short time, the condenser surface is coated with fine droplets. The H2S3 collects slowly but uniformly in receiver b, which is at room temperature, while the H2S2 is condensed in trap c, which is cooled with a Dry Ice-methanol mixture. Then, the bath temperature is slowly increased to 125°C over 20 minutes. The flask contents are cooled to 110°C, and 15 ml of H2Sx is again added through d. The 20-minute heating procedure is repeated. After two portions of H2Sx have been cracked, the vacuum is released, and the air is slowly introduced into the apparatus, passing through the CaCl3 and soda-lime tubes. The ground glass joint is then quickly disconnected, and the hot, still liquid residue is poured out, whereupon it solidifies. After the ground glass joint has been resealed, the cracking continues, and the residue is removed again after two 15-ml additions of H2Sx. About 25 ml of H2S3 and 15 ml of H2S2 are obtained from 120 ml of freshly prepared H2Sx. With aged H2Sx, the yield of H2S2 increases, while that of H2S3 is reduced. Both products are nearly pure; at most, each is contaminated by a small amount of the other.
SULPHIDE Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

