Alonacic
|
|
Sicherheit
Alonacic Chemische Eigenschaften,Einsatz,Produktion Methoden
Originator
Alonacic ,Onbio Inc.Manufacturing Process
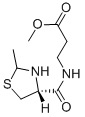
Preparation of (2-methylthiazolidin-4-carbonyl)-β-alanine methyl ester:To a solution of β-alanine methyl ester hydrochloride (5.08 g, 36.4 mmol) in dimethylformamide (35 ml) kept under stirring at -5°C, N-methylmorpholine (4.01 ml, 36.4 mmol) and then a solution of N-t-butoxycarbonyl-2- methylthiazolidine-4-carboxylic acid (9 g, 36.4 mmol) in dimethylformamide (15 ml) were added. To the resulting solution kept under stirring at -5°C, dicyclohexylcarbodiimide (9 g, 43.68 mmol) and N-hydroxybenzotriazole (5.89 g, 43.68 mmol) were added. After 24 hours under stirring at +4°C, the precipitate (dicyclohexylurea) was filtered and the filtrate was evaporated to dryness. An oil was obtained which was dissolved in ethyl acetate and the solution was washed with an aqueous solution of citric acid at 10%, with an aqueous sodium bicarbonate solution at 10% and with water. The organic solution, dried on sodium sulphate was evaporated to dryness under vacuum at 40°C. (N-t-Butoxycarbonyl-2-methylthiazolidine-4-carbonyl)-β-alanine methyl ester (10.7 g) was thus obtained as oil. The obtained product (7.9 g) was treated at room temperature under nitrogen, with ethyl acetate (90 ml) containing 13% (w/v) of hydrogen chloride. After 1 hour the solution was evaporated to dryness under vacuum at 35°C.
The residue, after crystallization from isopropyl alcohol diethyl ether, afforded (2-methylthiazolidine-4-carbonyl)-β-alanine methyl ester hydrochloride (5.4 g). [α]D20=-85° (c=1, CH3OH); MP:=124°-125°C. The base form may be prepared by adding of equivalent of any basic product (NaHCO3, Et3N and so on).
Therapeutic Function
MucolyticAlonacic Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Alonacic Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 0)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate |
|---|
- Alonacic
- 105292-70-4