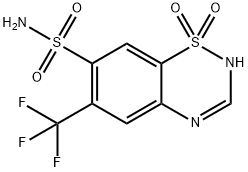
Flumethiazid
|
|
Flumethiazid Eigenschaften
- Schmelzpunkt:
- 306.6°C
- Siedepunkt:
- 560.0±60.0 °C(Predicted)
- Dichte
- 1.6578 (estimate)
- pka
- pKa 6.3(H2O) (Uncertain)
- Wasserl?slichkeit
- 1.05g/L(room temperature)
Sicherheit
| Giftige Stoffe Daten | 148-56-1(Hazardous Substances Data) |
|---|
Flumethiazid Chemische Eigenschaften,Einsatz,Produktion Methoden
Originator
Ademol,Squibb,US,1959Manufacturing Process
Chilled 3-trifluoromethylaniline (32.2 g) is added dropwise over a 45-minute period to 150 ml of chlorosulfonic acid with stirring and cooling. The ice bath is removed and 140 g of sodium chloride is added over 3 hours. The mixture is heated on a water bath for 30 minutes, then gradually up to 160°C over 6 hours. The cooled reaction mixture is diluted with 500 ml of an ice water slurry and taken into ether. The ether is dried and evaporated to leave 5- trifluoromethylamine-2,4-disulfonyl chloride.The crude residue is heated on the steam bath for 1 hour with 75 ml of concentrated ammonium hydroxide. Cooling and filtration gives 2,4- disulfamyl-5-trifluoromethylaniline, MP 241°C to 243°C.
This intermediate is treated with an excess of 98% formic acid at steam bath temperature for 3 hours. Evaporation and dilution with water gives 7-sulfamyl- 6-trifluoromethyl-1,2,4-benzothiadiazine-1,1-dioxide, MP 304°C to 308°C.
Therapeutic Function
Carbonic anhydrase inhibitorFlumethiazid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Flumethiazid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 3)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | |
support@targetmol.com | United States | 38630 | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 029-81120477 17792793610 |
1033@dideu.com | China | 9951 | 58 |
148-56-1(Flumethiazid)Verwandte Suche:
- 6-Trifluoromethyl-7-sulfamoyl-4H-1,2,4-benzothiazine 1,1-dioxide
- Ademol
- Fludemil
- Routrax
- Trifluoromethylthiazide
- flumethiazide
- NSC-44626
- NSC44626
- NSC 44626
- 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-(trifluoromethyl)-, 1,1-dioxide
- 148-56-1
- C8H6F3N3O4S2