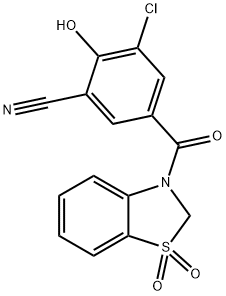
Dotinurad Impurity 9
|
|
Dotinurad Impurity 9 Eigenschaften
- Siedepunkt:
- 636.6±55.0 °C(Predicted)
- Dichte
- 1.70±0.1 g/cm3(Predicted)
- pka
- 3.28±0.25(Predicted)
Sicherheit
Dotinurad Impurity 9 Chemische Eigenschaften,Einsatz,Produktion Methoden
Dotinurad Impurity 9 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Dotinurad Impurity 9 Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 6)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Moxin Chemicals | +8617320513646 |
mike@molcoo.com | China | 9531 | 58 |
| QUALITY CONTROL SOLUTIONS LTD. | 0755-66853366; 13670046396 |
ORDERS@QCSRM.COM | China | 24342 | 58 |
| Hubei Yangxin Medical Technology Co., Ltd. | 15374522761 |
3003392093@qq.com | China | 8706 | 55 |
| Foshan Heshuo Pharm technology Co., ltd | 15363639686 |
heshuoyiyao@hotmail.com | China | 899 | 58 |
| Hubei Qingbei Yunyan Pharmaceutical Technology Co., Ltd | 18162595016; 18162595016 |
3287908757@qq.com | China | 9122 | 58 |
| ShenZhen H&D Pharmaceutical Technology Co., LTD | 18126517264 |
2850476883@qq.com | China | 7905 | 58 |
- Dotinurad Impurity 9
- Benzonitrile, 3-chloro-5-[(1,1-dioxido-3(2H)-benzothiazolyl)carbonyl]-2-hydroxy-
- 1285572-63-5