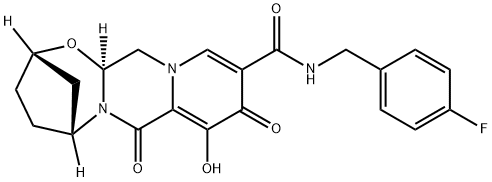
Bictegravir Impurity 3
|
|
Bictegravir Impurity 3 Eigenschaften
- Siedepunkt:
- 702.2±60.0 °C(Predicted)
- Dichte
- 1.54±0.1 g/cm3(Predicted)
- pka
- 4.50±1.00(Predicted)
Sicherheit
Bictegravir Impurity 3 Chemische Eigenschaften,Einsatz,Produktion Methoden
Bictegravir Impurity 3 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Bictegravir Impurity 3 Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 6)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Guangzhou Jiuzhi Pharmaceutical Technology Co., Ltd. | 020-89854820 |
sales@jzpharma.cn | China | 1130 | 58 |
| Shenzhen Roark Pharma-Tec Co., Ltd. | 13128912434 |
2880126944@qq.com | China | 2577 | 58 |
| guoyungurui | 18162595016; 18162595016 |
3287908757@qq.com | China | 10335 | 58 |
| Hubei Moco Chemical Co., Ltd. | 18627753421 18627756402 |
3001009495@qq.com | China | 19083 | 58 |
- Bictegravir Impurity 3
- (2S,5R,13aS)-N-(4-Fluorobenzyl)-8-hydroxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1'',2'':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxamide
- 2,5-Methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxamide, N-[(4-fluorophenyl)methyl]-2,3,4,5,7,9,13,13a-octahydro-8-hydroxy-7,9-dioxo-, (2S,5R,13aS)-
- Chlorfenvinphos Impurity 8
- Bikagwe Impurity 3
- 1616339-82-2