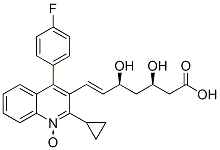
Pitavastatin Impurity 12
|
|
Sicherheit
Pitavastatin Impurity 12 Chemische Eigenschaften,Einsatz,Produktion Methoden
Pitavastatin Impurity 12 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Pitavastatin Impurity 12 Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 7)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Standardpharm Co. Ltd. | 86-714-3992388 |
overseasales1@yongstandards.com | United States | 14332 | 58 |
| QUALITY CONTROL SOLUTIONS LTD. | 0755-66853366; 13670046396 |
ORDERS@QCSRM.COM | China | 24342 | 58 |
| BOC Sciences | 1-631-485-4226; 16314854226 |
info@bocsci.com | United States | 12952 | 65 |
| Shenzhen SUNGENING Bio-Medical Co., Ltd. | 0755-28967200 13631290199 |
wxf@sungening.com | China | 9580 | 60 |
| Cato Research Chemicals Inc. | 020-81215950 4000-868-328 |
customer@uwalab.com | United States | 10404 | 58 |
| Changzhou Xianglong Pharmaceutical Technology Co., LTD | 18661161657 |
yaoxiangqiu2021@163.com | China | 10000 | 58 |
- Pitavastatin Impurity 12
- Pivastatin impurity 12