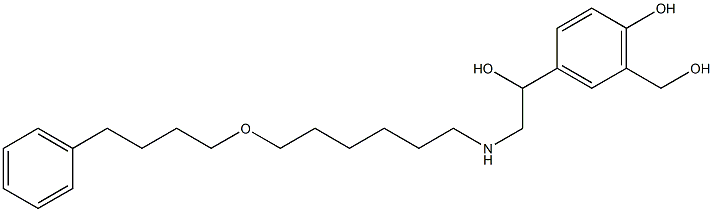
SalMeterol EP IMpurity G
|
|
SalMeterol EP IMpurity G Eigenschaften
- Siedepunkt:
- 912.8±65.0 °C(Predicted)
- Dichte
- 1.129±0.06 g/cm3(Predicted)
- storage temp.
- Refrigerator
- L?slichkeit
- Chloroform (Slightly), Methanol (Slightly)
- Aggregatzustand
- Solid
- pka
- 9.69±0.40(Predicted)
- Farbe
- Colourless to Off-White
Sicherheit
SalMeterol EP IMpurity G Chemische Eigenschaften,Einsatz,Produktion Methoden
Verwenden
Salmeterol Dimer Impurity is an impurity of the β2-Adrenergic agonist Salmeterol (S090100).SalMeterol EP IMpurity G Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
SalMeterol EP IMpurity G Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 51)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 |
overseasales1@yongstandards.com | United States | 14332 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29810 | 58 |
| sgtlifesciences pvt ltd | +8617013299288 |
dj@sgtlifesciences.com | China | 12373 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 |
masar@topule.com | China | 8467 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 |
jkinfo@jkchemical.com | China | 96815 | 76 |
| Chemsky (shanghai) International Co.,Ltd | 021-50135380 |
shchemsky@sina.com | China | 15402 | 60 |
| Daicel Chiral Technologies (China)CO.,LTD | 021-50460086-9 15921403865 |
han_yajun@dctc.daicel.com | China | 6791 | 65 |
| BOC Sciences | 1-631-485-4226; 16314854226 |
info@bocsci.com | United States | 14055 | 65 |
| Cato Research Chemicals Inc. | 020-81960175 |
min.he@cato-chem.com | China | 1955 | 55 |
1391051-88-9()Verwandte Suche:
- 1-[4-Hydroxy-3-[[[2-hydroxy-2-[4-hydroxy-3-(hydroxyMethyl)phenyl]ethyl][6-(4-phenylbutoxy)hexyl]aMino]Methyl]phenyl]2-[[6-(4-phenylbutoxy)hexyl]aMino]ethanol
- SalMeterol DiMer IMpurity
- SalMeterol EP IMpurity G
- Salmeterol Xinafoate BP Impurity G
- Salmeterol Impurity 8(Salmeterol EP Impurity G )
- Salmeterol Dimer Impurity (Mixture of Diastereomers)
- Salmeterol Impurity 8(EP Impurity G )
- Salmeterol?EP Impurity G/ Salmeterol N-Alkyl Impurity (Salmeterol Dimer Impurity)
- salmeterol impurities1069
- Salmeterol EP Impurity GQ: What is Salmeterol EP Impurity G Q: What is the CAS Number of Salmeterol EP Impurity G Q: What is the storage condition of Salmeterol EP Impurity G Q: What are the applications of Salmeterol EP Impurity G
- 4-(1-Hydroxy-2-((2-hydroxy-4-(1-hydroxy-2-((6-(4-phenylbutoxy)hexyl)amino)ethyl)benzyl)(6-(4-phenylbutoxy)hexyl)amino)ethyl)-2-(hydroxymethyl)phenol
- Salmeterol EP Impurity G / Salmeterol Dimer Impurity
- 4-(1-Hydroxy-2-((2-hydroxy-4-(1-hydroxy-2-((6-(4-phenylbutoxy)hexyl)amino)ethyl)benzyl)(6-(4-phenylbutoxy)hexyl)amino)ethyl)-2-(hydroxymethyl)phenol (Salmeterol Impurity)
- Salmeterol EP Impurity G (dimer)
- Salmeterol Impurity 20
- 4-[1-Hydroxy-2-[[2-hydroxy-4-[1-hydroxy-2-[6-(4-phenylbutoxy)hexylamino]ethyl]phenyl]methyl-[6-(4- phenylbutoxy)hexyl]amino]ethyl]-2-(hydroxymethyl)phenol
- 1391051-88-9
- C50H72N2O7
- Amines
- Aromatics
- Impurities
- Intermediates & Fine Chemicals
- Pharmaceuticals