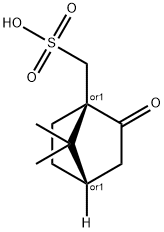
Voriconazole EP IMpurity E
|
|
Voriconazole EP IMpurity E Eigenschaften
- Dichte
- 1.331±0.06 g/cm3(Predicted)
- pka
- 1.17±0.50(Predicted)
Sicherheit
Voriconazole EP IMpurity E Chemische Eigenschaften,Einsatz,Produktion Methoden
Voriconazole EP IMpurity E Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Voriconazole EP IMpurity E Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 8)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hubei Ipure Biology Co., Ltd | +8613367258412 |
ada@ipurechemical.com | China | 10319 | 58 |
| TOSUN PHARM | 020-61855200 13326451905 |
260366801@qq.com | China | 7824 | 58 |
| Jinan blalong chemical co. LTD | 2710913286@.com |
1513643261@qq.com | China | 14246 | 58 |
| Changzhou Xianglong Pharmaceutical Technology Co., LTD | 0519-88910898 18661161657 |
yaoxiangqiu2021@163.com | China | 9996 | 58 |
| Molcoo Chemicals Inc. | 18576690171 17386083646 |
3001006472@qq.com | China | 11801 | 58 |
| Henan Vcommend Consultrading Co., Ltd. | 13393721940; 13393721940 |
sales@vcommend.com | China | 10005 | 58 |
| Hubei Moco Chemical Co., Ltd. | 18627753421 18627756402 |
3001009495@qq.com | China | 19077 | 58 |
- Voriconazole EP IMpurity E
- VoricozoleEPImpurityE
- Bicyclo[2.2.1]heptane-1-methanesulfonic acid, 7,7-dimethyl-2-oxo-, (1R,4S)-rel-
- 2287183-06-4