Scandium trifluoromethanesulfonate Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Beschreibung
Scandium trifluoromethanesulfonate, commonly called Scandium(III) triflate, is a chemical compound with formula Sc(SO3CF3)3, a salt consisting of scandium cations Sc3+ and triflate SO3CF3? anions.
Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′S,5′S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (E)-2-oxo-4-aryl-3-butenoates.
Chemische Eigenschaften
White powder
Verwenden
Scandium(III) trifluoromethanesulfonate is widely used as a catalyst in hydrothiolation, selective two-electron reduction of oxygen by ferrocene derivatives and vinylogous Fridel-crafts alkylation of indoles and pyrrole in water. It is involved in the Mukaiyama aldol addition and stereochemically catalyzes the radical polymerization of acrylates. It acts as a Lewis acid catalyst and used in the synthesis of bullvalone via a stabilized sulfur ylide.
Application
Scandium(III) triflate was used as a catalyst in:
Hydrothiolation reaction of aromatic and aliphatic thiols.
Selective two-electron reduction of O2 by ferrocene derivatives.
Vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water.
Synthesis of β-cyanoketones.
Combination with triethylsilane to reductively open functionalized pyranoside rings.
The key steps of synthesis of bullvalone via a stabilized sulfur ylide.
synthetische
Scandium triflate (Scandium trifluoromethanesulfonate) can be prepared from the corresponding oxide (Sc2O3) and aqueous trifluoromethanesulfonic acid (TfOH). After filtration and concentration of the clear aqueous solution in vacuo, the resulting hydrated salt is dried in vacuo (<1 mmHg) at 200?°C for 40 h to afford the anhydrous triflate stored over P2O5.
Reaktionen
-
-
Water tolerant Lewis acid.
-
-
Commonly used in a range of Lewis acid catalyzed reactions.
-
-
Efficient metal source for Lewis acid catalyzed asymmetric reactions.
-
-
Catalyzes Friedel-Crafts alkylation, acylation and related reactions.
-
-
Catalyzes various domino- and multi-component processes.
-
-
Catalyzes electrophilic additions of alpha-diazoesters with ketones.
-
-
Catalyzes carbon insertion reactions.
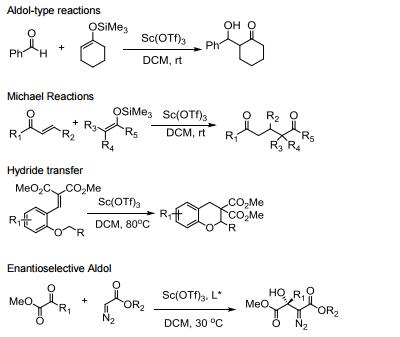
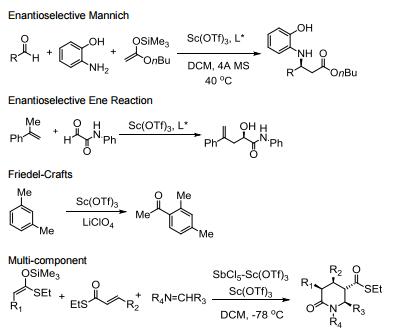
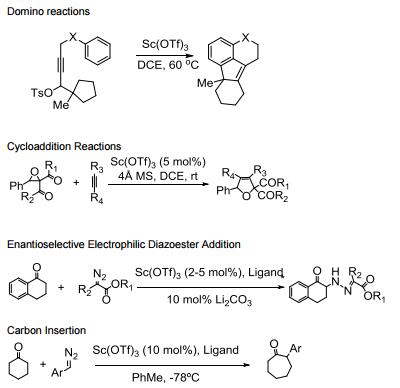
Allgemeine Beschreibung
Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′
S,5′
S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (
E)-2-oxo-4-aryl-3-butenoates.
Scandium trifluoromethanesulfonate Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

