Nalfurafine hydrochloride Chemische Eigenschaften,Einsatz,Produktion Methoden
Verwenden
Nalfurafine hydrochloride was launched on March of 2009 in Japan as the first in class non-narcotic opioid drug for intractable itch caused by hemodialysis. It showed significant opioid κ-agonist activity and induced neither aversion nor preference in rat
Clinical Use
The j opioid receptor agonist nalfurafine hydrochloride was approved
and launched in 2009 for the first time in Japan. Nalfurafine
is indicated for the treatment of pruritus in hemodialysis patients
who have not responded to conventional therapies. Hemodialysis related uremic pruritus is characterized by severe systemic itching
without inflammation of the skin. Its cause has not been fully
elucidated, and it often does not respond to treatment by conventional
antipruritic drugs, such as antihistamines. Nalfurafine was
co-developed by Toray, Japan Tobacco and Torii Pharmaceuticals
and manufactured and marketed by Toray. It has orphan drug
status in Japan for the approved indication.
Synthese
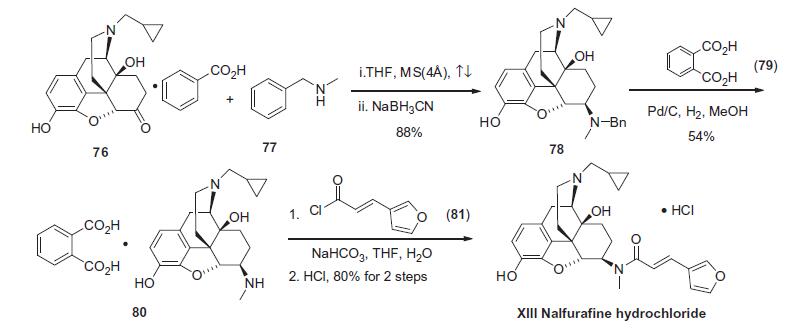
The preparation of
nalfurafine hydrochloride started with naltrexone benzoate (76)
which is available commercially from Aldrich. 76 was treated
with benzylmethylamine (77) in refluxing THF in the presence of
molecular sieves (4 Å) to form the intermediate imine which was
subsequently reduced in situ using sodium cyanoborohydride to
provide benzylmethyl amine 78 in 88% yield. Removal
of the benzyl group in 78 via catalytic hydrogenation and salting
the resulting amine with phthalic acid (79) gave secondary amine
80 in 54% yield. Phthalate 80 was then combined with trans-3-
(furyl)acryloyl chloride (81) and sodium bicarbonate in THF/water
to provide nalfurafine which was then treated with HCl to provide
nalfurafine hydrochloride (XIII) in 80% yield.
Nalfurafine hydrochloride Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

