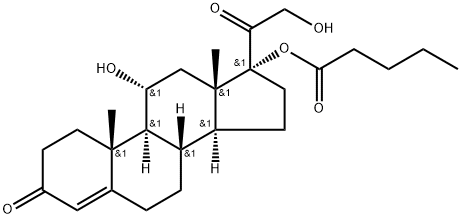
HYDROCORTISONE IMPURITY 11
|
|
HYDROCORTISONE IMPURITY 11 Eigenschaften
- Siedepunkt:
- 595.1±50.0 °C(Predicted)
- Dichte
- 1.21±0.1 g/cm3(Predicted)
- pka
- 12.98±0.10(Predicted)
Sicherheit
HYDROCORTISONE IMPURITY 11 Chemische Eigenschaften,Einsatz,Produktion Methoden
Verwenden
11-Epihydrocortisone Valerate is a derivative of Hydrocortisone (H714615) which is a glucocorticoid that is produced by the zona fasciculata of the adrenal gland.HYDROCORTISONE IMPURITY 11 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
HYDROCORTISONE IMPURITY 11 Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 4)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hubei Yangxin Medical Technology Co., Ltd. | 15374522761 |
3003392093@qq.com | China | 8705 | 55 |
| Jinan blalong chemical co. LTD | 2710913286@.com |
1513643261@qq.com | China | 14246 | 58 |
| Shanghai Zerui Biotechnology Co., Ltd. | 021-54580889 18321124657 |
731997554@qq.com | China | 10608 | 58 |
- HYDROCORTISONE IMPURITY 11
- Pregn-4-ene-3,20-dione, 11,21-dihydroxy-17-[(1-oxopentyl)oxy]-, (11α)- (9CI)
- Clascoterone Impurity 16
- 139755-29-6