4-(1-Naphthyl)-4-oxobutansaeure
|
|
|
- CAS-Nr.
- 3562-99-0
- Bezeichnung:
- 4-(1-Naphthyl)-4-oxobutansaeure
- Englisch Name:
- Menbutone
- Synonyma:
- menbutone;genabil;menbuton;fel-bis;Icteryl;SC 1749;naftobil;epanaftol;genabilin;Meng Meng
- CBNumber:
- CB3489226
- Summenformel:
- C15H14O4
- Molgewicht:
- 258.27
- MOL-Datei:
- 3562-99-0.mol
|
4-(1-Naphthyl)-4-oxobutansaeure Eigenschaften
- Schmelzpunkt:
- 172-173°
- Siedepunkt:
- 361.52°C (rough estimate)
- Dichte
- 1.2105 (rough estimate)
- Brechungsindex
- 1.4872 (estimate)
- storage temp.
- Sealed in dry,Room Temperature
- L?slichkeit
- DMSO (Slightly), Methanol (Slightly, Sonicated), Ethanol (Slightly, Heated)
- Aggregatzustand
- Solid
- pka
- 4.44±0.17(Predicted)
- Farbe
- White to Off-White
- InChI
- InChI=1S/C15H14O4/c1-19-14-8-6-11(13(16)7-9-15(17)18)10-4-2-3-5-12(10)14/h2-6,8H,7,9H2,1H3,(H,17,18)
- InChIKey
- FHGJSJFIQNQBCK-UHFFFAOYSA-N
- SMILES
- C12C=CC=CC1=C(OC)C=CC=2C(=O)CCC(=O)O
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Bildanzeige (GHS) |

|
| Alarmwort |
Warnung |
| Gefahrenhinweise |
| Code |
Gefahrenhinweise |
Gefahrenklasse |
Abteilung |
Alarmwort |
Symbol |
P-Code |
| H302 |
Gesundheitssch?dlich bei Verschlucken. |
Akute Toxizit?t oral |
Kategorie 4 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P270, P301+P312, P330, P501 |
| H315 |
Verursacht Hautreizungen. |
Hautreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P302+P352, P321,P332+P313, P362 |
| H319 |
Verursacht schwere Augenreizung. |
Schwere Augenreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P305+P351+P338,P337+P313P |
| H335 |
Kann die Atemwege reizen. |
Spezifische Zielorgan-Toxizit?t (einmalige Exposition) |
Kategorie 3 (Atemwegsreizung) |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
|
|
| Sicherheit |
| P261 |
Einatmen von Staub vermeiden. |
| P305+P351+P338 |
BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach M?glichkeit entfernen. Weiter spülen. |
|
4-(1-Naphthyl)-4-oxobutansaeure Chemische Eigenschaften,Einsatz,Produktion Methoden
Originator
Hepalande, Delalande ,W. Germany ,1977
Manufacturing Process
395 parts of (α-methoxynaphthalene and 265 parts of succinic anhydride are dissolved in 8,000 parts of dry benzene at room temperature. The resulting solution is stirred and 710 parts of anhydrous aluminum chloride are added over a period of twenty minutes. During the addition the temperature of the reaction mixture rises to about 60°C to 70°C. After the addition the reaction mixture is stirred for fifteen or twenty minutes at 60°C to 70°C and then refluxed for one hour. The hot reaction mixture is then poured onto a mixture of 5,000 parts of ice and 900 parts of concentrated hydrochloric acid. The benzene is removed by steam distillation and the hot aqueous residue is filtered to remove the insoluble β-(1-methoxy-4-naphthoyl)-propionic acid. The residue of the latter is dried and then dissolved in 16,000 parts of hot water containing 300 parts of sodium carbonate. The hot solution is treated with activated charcoal, filtered while hot, chilled and acidified. The residue of purified acid is collected on a filter, washed with water, and dried at 65°C. A yield of 552 parts of purified β-(1-methoxy)-4-naphthoyl)propionic acid, melting at 172°C to 173°C is obtained.
Therapeutic Function
Choleretic
Synthese
Menbutone is prepared by the reaction of succinic acid anhydride and 1-Methoxynaphthalene. The steps are as follows:
15.8 g of 1-methoxynaphthalene and 10.0 g of succinic anhydride were dissolved in 120 mL of dichloromethane,Stir, Cooling to 1 ~ 3 ° C, Divided into three batches of anhydrous aluminum trichloride 15.0 grams, The addition process takes about 20 minutes, The solution was then heated to 35 ± 2 ° C, Insulation reaction 6 hours (5.5 ~ 6.5 hours range), After the reaction is complete, The reaction solution was poured into an ice-water mixture (200 g of ice and 300 g of water) for 30 minutes,Standing, analysiscrystal, Filter, The filtrate was heated and distilled to recover dichloromethane, The cake is the crude of the ketone ketone; And then the crude ketoprofen water as a solvent by 2-3 times recrystallization, Activated carbon decolorization, Demon ketone boutique. The mass of the present product of the compound was 22.3 g, Melting point of 176 ~ 179 ° C, The yield was 86.4%.
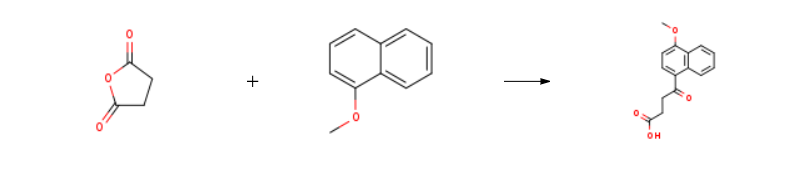
4-(1-Naphthyl)-4-oxobutansaeure Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
4-(1-Naphthyl)-4-oxobutansaeure Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 146)Lieferanten
3562-99-0(4-(1-Naphthyl)-4-oxobutansaeure)Verwandte Suche:
- genabilicacid
- genabilin
- ido-genabil
- naftobil
- 3-(4-methoxy-1-naphthoyl)-propionicaci
- 4-methoxy-gamma-oxo-1-naphthalenebutanoicaci
- acidebeta-(1-methoxy-4-naphthoyl)propionique
- beta-(1-methoxy-4-naphthoyl)-propionsaeure
- epanaftol
- fel-bis
- nafto-epatina
- P-METHOXYNAPHTHOYLPROPIONIC ACID
- 3-(4-METHOXY-1-NAPHTHOYL)PROPIONIC ACID
- Icteryl
- SC 1749
- 1-Methoxy-4-(.beta.-carboxyethylcarbonyl)-naphthalene
- 4-keto-4-(4-methoxy-1-naphthyl)butyric acid
- 4-(4-Methoxy-[1]naphthyl)-4-oxo-butyric acid
- 1-Naphthalenebutanoic acid, 4-methoxy-g-oxo-
- Meng Meng
- 3-(4'-METHOXYNAPHTHOYL) PROPIONIC ACID
- MENBUTONE STANDARD
- 1-Naphthalenebutanoic acid, 4-methoxy-γ-oxo-
- 3562-99-0 3-(4-METHOXY-1-NAPHTHOYL)PROPIONIC ACID C15H14O4
- genabil
- menbuton
- menbutone
- Meng cloth ketone
- Mengbuketone 3562-99-0
- Menbutone, 10 mM in DMSO
- 3562-99-0
- Aromatic Propionic Acids

