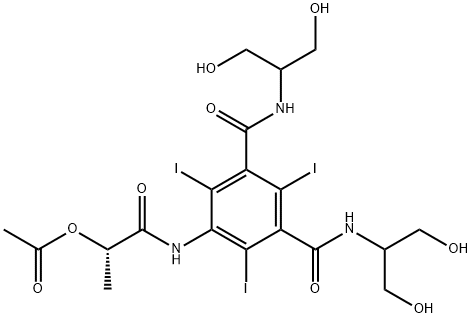
Iopamidol EP impurity E
|
|
Iopamidol EP impurity E Eigenschaften
- Siedepunkt:
- 788.4±60.0 °C(Predicted)
- Dichte
- 2.157±0.06 g/cm3(Predicted)
- pka
- 10.28±0.70(Predicted)
Sicherheit
Iopamidol EP impurity E Chemische Eigenschaften,Einsatz,Produktion Methoden
Verwenden
Iopamidol EP Impurity E is an impurity of and is used to synthesize Iopamidol (I735600).Iopamidol EP impurity E Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Iopamidol EP impurity E Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 36)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Standardpharm Co. Ltd. | 86-714-3992388 |
overseasales1@yongstandards.com | United States | 14332 | 58 |
| Xilinglab Co., Ltd. | +8618381337976 |
bd@xilinglab.com | China | 86 | 58 |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 |
overseasales@yongstandards.com | China | 11922 | 58 |
| QUALITY CONTROL SOLUTIONS LTD. | 0755-66853366; 13670046396 |
ORDERS@QCSRM.COM | China | 24342 | 58 |
| Guangzhou PI PI Biotech Inc | 020-81716320 +86-13602409664 |
Sales@pipitech.com | China | 3633 | 55 |
| BOC Sciences | 1-631-485-4226; 16314854226 |
info@bocsci.com | United States | 14055 | 65 |
| ChemStrong Scientific Co.,Ltd | 0755-0755-66853366 13670046396 |
sales@chem-strong.com | China | 18088 | 56 |
| Shanghai Kewel Chemical Co., Ltd. | 021-64609169 18901607656 |
greensnown@163.com | China | 9906 | 50 |
| Shanghai Xiang Xin Industrial Co., Ltd. | 021-50211651 13651607026 |
sales@xxgmpharm.com | China | 481 | 58 |
| BOC Sciences | 16314854226 |
info@bocsci.com | United States | 9923 | 65 |
60166-92-9()Verwandte Suche:
- Iopamidol EP impurity E
- Iopamidol Impurity 5(Iopamidol EP Impurity E)
- 1,3-Benzenedicarboxamide, 5-[[(2S)-2-(acetyloxy)-1-oxopropyl]amino]-N,N'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo- (9CI)
- 5-?[[(2S)?-?2-?(Acetyloxy)?-?1-?oxopropyl]?amino]?-?N,?N&rsquo
- -?bis[2-?hydroxy-?1-?(hydroxymethyl)?ethyl]?-?2,?4,?6-?triiodo-1,?3-?benzenedicarboxamide?(IopamidolEPImpurityE)
- 5-?[[(2S)?-?2-?(Acetyloxy)?-?1-?oxopropyl]?amino]?-?N,?N'-?bis[2-?hydroxy-?1-?(hydroxymethyl)?ethyl]?-?2,?4,?6-?triiodo-1,?3-?benzenedicarboxamide? (Iopamidol EP Impurity E)
- (1S)-2-[[3,5-bis[[2-Hydroxy-1-(hydroxymethyl)ethyl]carbamoyl]-2,4,6-triiodophenyl]amino]-1-methyl-2-oxoethyl acetate
- 1,3-Benzenedicarboxamide, 5-[[(2S)-2-(acetyloxy)-1-oxopropyl]amino]-N,N′-bis[2-hydroxy-1-(hydroxymet
- 1,3-Benzenedicarboxamide, 5-[[(2S)-2-(acetyloxy)-1-oxopropyl]amino]-N,N′-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo
- (S)-1-((3,5-Bis((1,3-dihydroxypropan-2-yl)carbamoyl)-2,4,6-triiodophenyl)amino)-1-oxopropan-2-yl acetate (Iopamidol Impurity)
- Iopanol EP Impurity E
- 60166-92-9
- C19H24I3N3O9