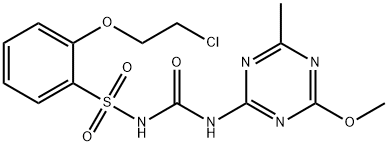
Triasulfuron
|
|
Triasulfuron Eigenschaften
- Schmelzpunkt:
- 178°C
- Siedepunkt:
- 150-260 °C
- Dichte
- 1.5218 (rough estimate)
- Dampfdruck
- 0Pa at 25℃
- Brechungsindex
- 1.5630 (estimate)
- storage temp.
- 0-6°C
- L?slichkeit
- DMSO (Slightly), Methanol (Slightly, Heated)
- pka
- 4.34±0.10(Predicted)
- BRN
- 7151883
- LogP
- 1.6-2.6 at 25℃ and pH5-9
- Dissoziationskonstante
- 4.64 at 20℃
- CAS Datenbank
- 82097-50-5(CAS DataBase Reference)
- EPA chemische Informationen
- Triasulfuron (82097-50-5)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | N | ||
|---|---|---|---|
| R-S?tze: | 50/53 | ||
| S-S?tze: | 60-61 | ||
| RIDADR | UN3077 9/PG 3 | ||
| WGK Germany | 2 | ||
| RTECS-Nr. | DB1554000 | ||
| HS Code | 29350090 | ||
| Giftige Stoffe Daten | 82097-50-5(Hazardous Substances Data) | ||
| Toxizit?t | LD50 in rats (mg/kg): >5000 orally; >2000 dermally; LC50 (4 hr) in rats: >5185 mg/m3 by inhalation (Amrein, Gerber) |
| Bildanzeige (GHS) |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | |||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||
| Sicherheit |
|
Triasulfuron Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R50/53:Sehr giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.S-S?tze Betriebsanweisung:
S60:Dieses Produkt und sein Beh?lter sind als gef?hrlicher Abfall zu entsorgen.S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
Verwenden
Herbicide.Definition
ChEBI: An N-sulfonylurea that is N-[o-(2-chloroethoxy)phenyl]sulfonylurea in which one of the hydrogens attached to the non-sulfonylated nitrogen has been replaced by a 4-methoxy-6-methyl-1,3,5-triazin-2-yl group A herbicide used to control broad-leaved weeds in cereals, its use within the EU has been banned after September 2017 on the grounds of potential groundwater contamination and risks to aquatic life.Landwirtschaftliche Anwendung
Herbicide: Used for the control of annual ryegrass, paradoxa grass and a wide range of broadleaf weeds in wheat and the post-emergence control of wild radishes in wheat, oats and barley. Registered for use in EU countries . Registered for use in the U.SHandelsname
AMBER®; CGA 131036®; LOGRAN®; RAVE®Stoffwechselwegen
Triasulfuron undergoes hydrolytic degradation, especially under acidic conditions, giving rise to cleavage of the sulfonylurea linkage, producing the major products 2-(2-chloroethoxy)benzenesulfonamide and 4-methyl-6-methoxy-2-amino-1,3,5-triazine and the minor product acetyltriuret. In plants, hydroxylation occurs at the 5-position of the phenyl ring to yield 5- hydroxytriasulfuron as a primary metabolite which is a major metabolite from the plant microsomal system.By mammals, orally administered triasulfuron is mainly excreted in the urine, and O-dealkylation of 2- chloroethoxy and 6-methoxy groups, and hydroxylation at the 5-position of the phenyl ring and at the 4-methyl group of the triazine ring are observed. By UV irradiation, 2-chloroethoxybenzene is interestingly identified with (4-methoxyl-6-methyl-1,3,5-triazine)urea, and, in sunlight, with these two products, 2-amino-4- methoxyl-6-methyltriazine and 2-(2- chloroethoxy)benzenesulfonamide are identified.
Triasulfuron Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Triasulfuron Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 172)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 |
aroma@qdtrustagri.com | China | 301 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29858 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49732 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12809 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17365 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 |
info@dycnchem.com | China | 52849 | 58 |
| changzhou huayang technology co., ltd | +8615250961469 |
2571773637@qq.com | China | 9691 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 |
sales@tnjchem.com | China | 25356 | 58 |
82097-50-5()Verwandte Suche:
Benoxacor
Methyl-4-hydroxybenzoat
Acetonitril
Chlorpropamid
Kresoxim-methyl
Paraquat-dichlorid
Methanol
Procarbazone sodium
Tolbutamid
4-Methoxyphenylaceton
Methylacrylat
Basic Violet 1
2-Methoxy-ethanol
Tribenuron methyl
Methyl
Ethametsulfuron
Herbicide
Benzolsulfonamid
- yl)amino)carbonyl)-
- 2-(2-chloroethoxy)-N-(((4-methyoxy-6-methyl-1,3,5-triazin-2-yl)amino)carbonyl)benzensulfonamide
- TRIASULFURON, 100MG, NEAT
- TRIASULFURON PESTANAL, 250 MG
- TRIASULFURON PESTANAL
- triasulfuron (bsi,draft-iso)
- TRIASULPHURON
- TRISULFURON
- triasulfuron 1-[2-(2-chloroethoxy)phenylsulfonyl]-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)urea
- Benzenesulfonamide, 2-(2-chloroethoxy)-N-[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl]-
- 1-(4-Methyl-6-methoxy-1,3,5-triazine-2-yl)-3-[[2-(2-chloroethoxy)phenyl]sulfonyl]urea
- 1-[2-(2-Chloroethoxy)phenylsulfonyl]-3-[(6-methoxy-4-methyl-1,3,5-triazin)-2-yl]urea
- 2-(2-chloroethoxy)-N-((4-Methoxy-6-Methyl-1,3,5-triazin-2-yl)carbaMoyl)benzenesulfonaMide
- Triasulfuron 0
- AMBER(R)
- 2-(2-chloroethoxy)-n-(((4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino)carbonyl)benzenesulfonamide
- 1-[2-(2-CHLOROETHOXY)PHENYLSULFONYL]-3-(4-METHOXY-6-METHYL)-(1,3,5-TRIAZIN-2-YL)UREA
- LOGRAN
- LOGRAN(R)
- Herbicide Safener Benoxacor
- cga-131036
- N-(6-methoxy-4-methyl-1,3,5-triazin-2-yl-aminocarbonyl)-2-(2-chloroethoxy)-benzenesulfonamide
- TRIASULFURON
- 1-(2-(2-chloroethoxy)phenylsulfonyl)-3-(4-methoxy-6-methyl-1,3,5-triazin-2-y
- 2-(2-chloroethoxy)-n-(((4-methoxy-6-methyl-1,3,5-triazin-2-benzenesulfonamid
- (1S,2R)-2-benzoyl-1-cyclohexanecarboxylic acid
- Triasulfuron Standard
- Triasulfuron@100 μg/mL in Acetonitrile
- Triasulfuron@1000 μg/mL in Acetonitrile
- Ethylbenzuron technical
- Triasulfuron PESTANAL(R), analytical standard
- C17648000 Triasulfuron
- Triasulfuron 100 μg/ml Acetonitrile
- Triasulfuron in Acetonitrile
- Biotin Impurity 46
- 82097-50-5
- C14H16ClN5O5S
- C14H16N5O5ClS
- Alphabetic
- TP - TZPesticides&Metabolites
- Alpha sort
- Herbicides
- Pesticides&Metabolites
- Q-ZAlphabetic
- Urea structure