(Diethoxymethyl)diphenylphosphine oxide Chemische Eigenschaften,Einsatz,Produktion Methoden
Verwenden
(Diethoxymethyl)diphenylphosphine Oxide is a readily accessible Horner-Wittig reagent for the conversion of aldehydes and ketones into homologous O,O-ketene acetals.
[1]
synthetische
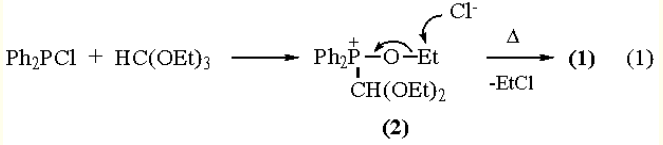
(Diethoxymethyl)diphenylphosphine Oxide (1) can be conveniently prepared by reaction of Chlorodiphenylphosphine with Triethyl Orthoformate. [2] Adduct (2) is formed in a fast, exothermic, step. Upon heating, an Arbuzov reaction occurs to give the
phosphine oxide (eq 1). Other O,O-acetals of formyldiphenylphosphine oxide can be prepared equally well. The usual procedure is for chlorodiphenylphosphine (88.2 g, 0.4 mol) to be added dropwise to triethyl orthoformate (59.3 g, 0.4 mol) over a period of 45 min. Subsequent heating of the reaction mixture (2 h, 110 °C) leads to formation of 24 g
(93% of theory) of ethyl chloride. Upon cooling, a practically quantitative yield of (1) is obtained, mp 74-77 °C. Addition of some solvent, when (1) starts precipitating, avoids the formation of a solid cake that is hard to remove from the reaction
flask. Crystallization from benzene/petroleum ether (80-110) affords the pure phosphine oxide.
(Diethoxymethyl)diphenylphosphine oxide Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

