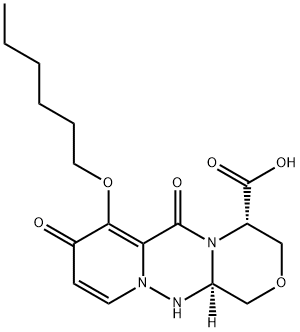
Baloxavir Impurity 70
|
|
Baloxavir Impurity 70 Eigenschaften
- Siedepunkt:
- 609.1±65.0 °C(Predicted)
- Dichte
- 1.40±0.1 g/cm3(Predicted)
- pka
- 3.22±0.40(Predicted)
Sicherheit
Baloxavir Impurity 70 Chemische Eigenschaften,Einsatz,Produktion Methoden
Baloxavir Impurity 70 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Baloxavir Impurity 70 Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 3)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| ShenZhen H&D Pharmaceutical Technology Co., LTD | +8618627948422 |
sale@hdimpurity.com | China | 3473 | 58 |
| Molcoo Chemicals Inc. | 18576690171 |
3001025734@qq.com | China | 11429 | 58 |
| ShenZhen H&D Pharmaceutical Technology Co., LTD | 18672472998 |
sunchenjie@hdimpurity.com | China | 7922 | 58 |
- Baloxavir Impurity 70
- 1H-[1,4]Oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazine-4-carboxylic acid, 7-(hexyloxy)-3,4,6,8,12,12a-hexahydro-6,8-dioxo-, (4S,12aR)-
- Baloxavir Marboxil Impurity 70
- 2305694-60-2