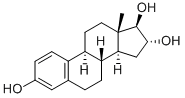
oestriol
|
|
oestriol Eigenschaften
- Schmelzpunkt:
- 280-282 °C(lit.)
- alpha
- D25 +58° ±5° (0.04 g in 1 ml dioxane)
- Siedepunkt:
- 370.61°C (rough estimate)
- Dichte
- 1.27
- Brechungsindex
- 58 ° (C=0.4, Dioxane)
- Flammpunkt:
- 9℃
- storage temp.
- 2-8°C
- L?slichkeit
- Practically insoluble in water, sparingly soluble in ethanol (96 per cent).
- Aggregatzustand
- Solid
- pka
- pKa 10.38±0.02(H2O t=23±2 Iunspeci?ed) (Uncertain)
- Farbe
- White to Off-White
- Optische Aktivit?t
- +6127 (95% ethanol)
- Wasserl?slichkeit
- 3.2mg/L(25 ºC)
- Merck
- 3707
- BRN
- 2508172
- InChIKey
- PROQIPRRNZUXQM-ZXXIGWHRSA-N
- CAS Datenbank
- 50-27-1(CAS DataBase Reference)
- NIST chemische Informationen
- Estriol(50-27-1)
- EPA chemische Informationen
- Estriol (50-27-1)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | T,F | ||
|---|---|---|---|
| R-S?tze: | 60-61-40-45-39/23/24/25-23/24/25-11 | ||
| S-S?tze: | 53-22-36/37/39-45-36/37-16 | ||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | KG8225000 | ||
| F | 8-10 | ||
| HS Code | 29072990 | ||
| Giftige Stoffe Daten | 50-27-1(Hazardous Substances Data) | ||
| Toxizit?t | LD50 oral in rat: > 2gm/kg |
| Bildanzeige (GHS) |

|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||
| Sicherheit |
|
oestriol Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R60:Kann die Fortpflanzungsf?higkeit beeintr?chtigen.R61:Kann das Kind im Mutterleib sch?digen.
R40:Verdacht auf krebserzeugende Wirkung.
R45:Kann Krebs erzeugen.
S-S?tze Betriebsanweisung:
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.S22:Staub nicht einatmen.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
Beschreibung
Estriol is significantly less active than estradiol; however, it has a selective ability to stimulate blood flow and restoration of genital epithelium. In addition, using this drug reduces mental symptoms of menopausal syndrome. It is used in the premenopausal and menopausal periods, for skin atrophy and signs of genital degeneration, and so on.Chemische Eigenschaften
White or almost white, crystalline powder.Verwenden
A metabolite of Estradiol. An estrogenic metabolite considerably less potent than the hormone EstradiolDefinition
ChEBI: A 3-hydroxy steroid that is estra-1,3,5(10)-trien-3-ol substituted by additional hydroxy groups at positions 16 and 17 (16alpha,17beta-stereoisomer).Allgemeine Beschreibung
Estriol, estra-1,3,5(10)-triene-3,16 ,17 -triol, is available for compounding into several differentformulations for use in HRT. It can be used alone or in combinationswith estradiol (Bi-Est) or with estradiol and estrone(Tri-Est).Hazard
A carcinogen (OSHA).Sicherheitsprofil
Suspected carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Other experimental reproductive effects. Mutation data reported. A steroid drug for the treatment of menopause. When heated to decomposition it emits acrid smoke and irritating fumes.l?uterung methode
Crystallise estriol from EtOH/ethyl acetate. Also purify it by countercurrent distribution with cyclohexane/EtOAc (1:1) and EtOH/H2O (1:1). The UV (EtOH) has max at 280nm ( 2,090 M-1cm-1). [Huffmann & Lott 71 719 1949, Leeds et al. J Am Chem Soc 76 2943 1954, Beilstein 6 IV 7550.]oestriol Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Estron
2,4-Dibromo-β-D-Glucopyranosiduronic Acid Estrane Derivative
3,16α-Dihydroxyestra-1,3,5(10)-trien-17-on-3,16-diacetat
16α,17α-Epoxy-1,3,5(10)-estratriene-3,17β-diol diacetate
1,3,5[10]-ESTRATRIENE-3,16ALPHA-DIOL-17-ONE
Epiestriol
2,4,16a-Tribromoestrone
2,4-Dibromo-16a-hydroxyestrone
Estra-1,3,5(10),16-tetraen-3,17-dioldiacetat
17-BETA-ESTRADIOL 3-METHYL ETHER
Downstream Produkte
oestriol Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 595)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hebei Bonster Technology Co.,Limited | +8613315996897 |
bsterltd.wendy@gmail.com | China | 792 | 58 |
| Hubei Lange Biotechnology Co., Ltd. | +8617762501948 |
sales2@langebiotech.com | China | 153 | 58 |
| Wuhan senwayer century chemical Co.,Ltd | +undefined-27-86652399 +undefined13627115097 |
market02@senwayer.com | China | 917 | 58 |
| Shenzhen Monkono Technology Co.,Ltd | +86-17063441314 |
sansbiotech@outlook.com | China | 677 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 |
eric@witopchemical.com | China | 23541 | 58 |
| Shandong Hanjiang Chemical Co., Ltd | +86-0533-2066820 +8618369939125 |
hanson@sdhanjiang.com | China | 1009 | 58 |
| Hebei Miaoyin Technology Co.,Ltd | +86-17367732028 +86-17367732028 |
kathy@hbyinsheng.com | China | 3512 | 58 |
| Shaanxi Xianhe Biotech Co., Ltd | +8617709210191 |
Jerry@xhobio.com | China | 882 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12830 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 |
allison@yan-xi.com | China | 5856 | 58 |
50-27-1(oestriol)Verwandte Suche:
- Overstin
- Ovesterin
- Ovestinon
- Ovestrion
- Ovo-vinces
- Stiptanon
- Synapause
- Thulol
- Trihydroxyestrin
- Trihydroxyoestrin
- Triodurin
- Triovex
- THEELOL
- OESTRIOL (E3)
- Estriol, Micronized (Plant Base), USP
- (1S,10R,11S,13R,14R,15S)-15-Methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2,4,6-triene-5,13,14-triol
- FeMale three alcohol
- Estriols
- (16-alpha,17-beta)-estra-1,3,5(10)-triene-3,16,17-triol
- (16-alpha,17-beta)-oestra-1,3,5(10)-triene-3,16,17-triol
- (16alpha,17beta)-Oestra-1,3,5(10)-triene-3,16,17-triol
- 1,3,5-estratriene-3-beta,16-alpha,17-beta-triol
- 1,3,5-Estratriene-3beta,16-alpha,17-beta-triol
- 1,3,5-oestratriene-3-beta,16-alpha,17-beta-triol
- 1,3,5-Oestratriene-3-beta,16alpha,17beta-triol
- 16-alpha,17-beta-estriol
- 16alpha,17beta-Estriol
- 3,16alpha,17beta-Trihydroxyestra-1,3,5(10)-triene
- 3,16-alpha,17-beta-Trihydroxyoestra-1,3,5(10)-triene
- 5(10)-triene-3,16,17-triol,(16alpha,17beta)-estra-3
- A 13610
- Aacifemine
- Colpogyn
- Colpovister
- Destriol
- Deuslon A
- deuslon-a
- Estra-1(10),2,4-triene-3,16,17-triol
- Estra-1,3,5(10)-trien-3,16alpha,17beta-triol
- Estra-1,3,5(10)-triene-3,16,17-triol, (16alpha,17beta)-
- Estra-1,3,5(10)-triene-3,16-alpha, l7-beta-triol
- estra-1,3,5(10)-triene-3,16-alpha,l7-beta-triol
- Estratriol
- Estriolo
- Folicular hormone
- Follicular hormone hydrate
- follicularhormonehydrate
- Gynaesan
- Gynasan
- Hemostyptanon
- 1,3,5(10)-Estratriene-3,16α,17β-triol
- 16α-Hydroxyestradiol
- (16a,17b)-Estra-1,3,5[10]-triene-3,16,17-triol
- Tricovex
- ESTRIOL,MICRONIZED,USP
- (8S,9S,13S,14S,16R,17R)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,16,17-triol
- ESTRIOL(RG)
- 13β-Methyl-1,3,5(10)-gonatriene-3,16α,17β-triol