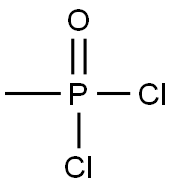
Methylphosphondichlorid
|
|
Methylphosphondichlorid Eigenschaften
- Schmelzpunkt:
- 31-34 °C
- Siedepunkt:
- 163 °C(lit.)
- Dichte
- 1.468 g/mL at 25 °C(lit.)
- Dampfdruck
- 760 mm Hg ( 163 °C)
- Flammpunkt:
- >110°C
- L?slichkeit
- Acetonitrile (Slightly), Chloroform
- Aggregatzustand
- solid
- Wasserl?slichkeit
- Soluble in water.
- Sensitive
- Moisture Sensitive
- BRN
- 1071305
- Stabilit?t:
- Stable, but may decompose in the presence of water or moisture. Incompatible with water, strong bases, strong oxidizing agents.
- CAS Datenbank
- 676-97-1
- EPA chemische Informationen
- Methylphosphonic dichloride (676-97-1)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | T+ | ||
|---|---|---|---|
| R-S?tze: | 14-26-34 | ||
| S-S?tze: | 26-36/37/39-45 | ||
| RIDADR | UN 3390 6.1/PG 1 | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | TA1840000 | ||
| F | 10 | ||
| TSCA | Yes | ||
| HazardClass | 6.1 | ||
| PackingGroup | I | ||
| Giftige Stoffe Daten | 676-97-1(Hazardous Substances Data) |
| Bildanzeige (GHS) |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||
| Sicherheit |
|
Methylphosphondichlorid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R14:Reagiert heftig mit Wasser.R26:Sehr giftig beim Einatmen.
R34:Verursacht Ver?tzungen.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
Chemische Eigenschaften
Methyl phosphonic dichloride is a low melting solid or colorless to pale yellow liquid. Pungent odor.Verwenden
suzuki reactionVorbereitung Methode
The oxidation of lowvalency organophosphorus compounds is important mainly in the case of the thiophosphonic acid chlorides. This method is used in the production of methylphosphonic acid dichloride by reaction of methyldichlorophosphine with sulfuryl chloride or chlorosulfuric acid. Elemental sulfur reacts with MePCl2 in the presence of catalytic quantities of tetraalkylphosphonium salts to form methylthiophosphonic acid dichloride.Allgemeine Beschreibung
Strongly irritates skin. Contact may destroy or irreversibly alter skin tissue. Very toxic by ingestion, inhalation, or by skin absorption. Combustible, though may be difficult to ignite.Air & Water Reaktionen
Fumes in moist air to form hydrogen chloride. Reacts with water to form hydrochloric acid, reaction may be violent.Reaktivit?t anzeigen
METHYLPHOSPHONIC DICHLORIDE is incompatible with water, strong oxidizing agents, alcohols, bases (including amines).. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].Health Hazard
Poisonous if inhaled or swallowed. Contact causes severe burns to skin and eyes.Brandgefahr
METHYLPHOSPHONIC DICHLORIDE may burn but does not ignite readily. May ignite other combustible materials (wood, paper, oil, etc.). Reacts violently with water. Flammable poisonous gases may accumulate in tanks and hopper cars. Runoff to sewer may create fire or explosion hazard. Contact causes severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution. Violent reaction with water.Sicherheitsprofil
Poison by inhalation. A corrosive irritant to the eyes, skin, and mucous membranes. When heated to decomposition it emits toxic fumes of Cland POx.m?gliche Exposition
Highly flammable; mists or Vapors may form explosive mixture with air. Reacts with moist air forming fumes of hydrogen chloride; may spontaneously ignite. Reacts with water or alcohol, forming hydrochloric acid. The reaction may be violent and ignite unreacted material. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, amines, ethers. May react violently, possibly explosively, when mixed with ethers and trace amounts of metal salts.Versand/Shipping
UN9206 Methyl phosphonic dichloride, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. Domestic (United States), Inhalation Hazard Zone B. UN3390 Toxic by inhalation liquid, corrosive, n.o.s. with an LC50 # 1000 mL/m3 and saturated vapor concentration ≥ 10 LC50 Hazard Class: 6.1; Labels: 6.1- Poisonous materials, 8-Corrosive material, Technical Name Required, Inhalation Hazard Zone Bl?uterung methode
Methylphosphonic dichloride [676-97-1] M 132.9, m 33o, 33-37o, b 53-54o/10mm, 64-6 7o/20.5mm, 86o/44mm, 162o/760mm, d 4 1.4382. Fractionally redistil it until the purity as checked by hydrolysis and acidimetry for Clis correct and the distillate should solidify on cooling. [Kinnear & Perren J Chem Soc 3437 1952, Crofts & Kosolapoff J Am Chem Soc 75 3379 1952, for IR see McIvor et al. Can J Chem 34 1611 1956, Beilstein 4 IV 3509.]Waste disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observedMethylphosphondichlorid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
676-97-1(Methylphosphondichlorid)Verwandte Suche:
methylphosphoniges Saeuredichlorid
Phenylphosphonsaeuredichlorid
Ethylphosphondichlorid
Propylphosphondichlorid
Methylphosphondichlorid
Octylphosphondichlorid
(Chlormethyl)phosphondichlorid
(2-Chlorethyl)phosphondichlorid
- dichloromethylphosphineoxide
- Methanephosphonic acid dichloride
- methanephosphonodichloridicacid
- methanephosphonylchloride
- methylphosphonicaciddichloride
- methyl-phosphonicaciddichloride
- methyl-phosphonicdichlorid
- methylphosphonyldichloride
- Methyl-phosphoramidedichloride
- NA 9206
- Phosphonic dichloride, methyl-
- Phosphonodichloridic acid, methyl-
- METHYLPHOSPHONIC DICHLORIDE
- METHANEPHOSPHONIC DICHLORIDE
- Methylphosphonicdichloride,98%
- methylphosphonoyl dichloride
- Methyldichlorophosphine oxide
- dichlorophosphorylmethane
- Methanephosphonyl dichloride
- CH3POCl2
- Methanephosphonic dichloride,Dichloromethylphosphine oxide,,Methanephosphonyl chloride,,Methylphosphonodichloridic acid, Methylphosphonyl dichloride
- Phosphonic dichloride,P-Methyl-
- methylphosphonodichloridicacid
- Methylphosphonsαuredichlorid
- methylphosphonylchloride
- methylpho
- Methylphosphonyl dichloride
- 676-97-1
- Phosphorus Precursors
- Phosphorus Compounds
- Catalysis and Inorganic Chemistry
- Catalysis and Inorganic Chemistry
- Phosphorus Compounds
- Phosphorus Precursors