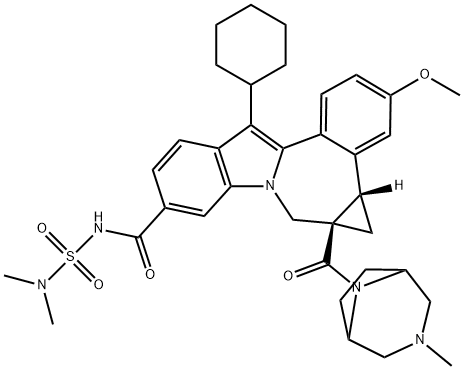
Beclabuvir
|
|
Beclabuvir Eigenschaften
- Dichte
- 1.45±0.1 g/cm3(Predicted)
- storage temp.
- Store at -20°C
- L?slichkeit
- DMSO:30.0(Max Conc. mg/mL);45.46(Max Conc. mM)
- Aggregatzustand
- Solid
- pka
- 4.44±0.40(Predicted)
- Farbe
- White to off-white
Sicherheit
Beclabuvir Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Beclabuvir is a non-nucleoside, nonstructural protein 5B (NS5B) polymerase inhibitor approved in Japan as part of a fixed-dose combination product for the treatment of hepatitis C virus (HCV). Upon administration and after intracellular uptake, the drug binds to the allosteric, noncatalytic “Thumb 1” site of NS5B resulting in a decreased rate of viral RNA synthesis and replication.4 Beclabuvir is combined with asunaprevir and declatasvir (both approved in 2014) and was discovered and developed by Bristol-Myers Squibb.Synthese
The syntheses of asunaprevir and declatasvir were described in an earlier review article.Condensation of indole-6-carboxylic acid (1) with cyclohexanone under basic conditions gave acid 2 in quantitative yield. Hydrogenation of the double bond in 2 using Pearlman?ˉs catalyst was followed by esterification to give ester 3 in high yield. Bromination of the indole at the 2-position was accomplished with pyridinium tribromide, and this was followed by saponification to provide acid 4. Treatment of 4 with carbonyldiimidazole (CDI) followed by N,N- dimethylsulfamide and 1, 8 - diazabicyclo[5.4.0]undec-7-ene (DBU) gave compound 5 in 74% yield. Suzuki coupling of 5 with commercial boronic acid 6 provided intermediate 7, which converted to hemiaminal 8 upon continued heating in 61% yield. Compound 8 was then treated with methyl 2-(dimethoxyphosphoryl)acrylate (9) to affect a tandem conjugate addition and Horner?Wadsworth? Emmons (HWE) olefination to give ester 10. Alternatively, the Suzuki coupling reaction of 5 with 6 could be stopped at intermediate 7, which could be treated with 9 to promote the tandem conjugate addition/HWE to give 10. Corey?-Chaykovsky cyclopropanation of 10 using sodium hydride and trimethylsulfoxonium iodide followed by chiral separation provided cyclopropane 11 in good yield and >99% enantiomeric excess (ee). Saponification of the methyl ester of 11 followed by coupling with 3-methyl-3,8-diazabicyclo[3.2.1]- octane dihydrochloride (12) gave beclabuvir (I) in high yield.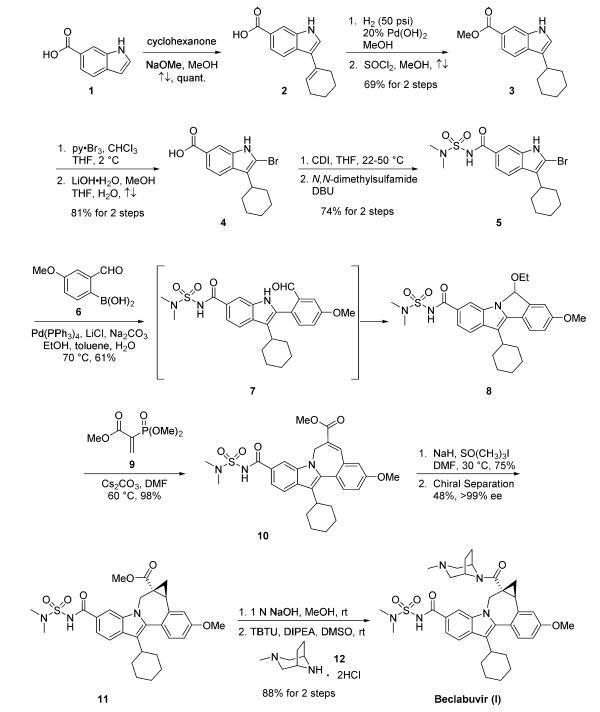
Beclabuvir Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Beclabuvir Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 33)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32161 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 |
sales@invivochem.cn | United States | 6391 | 58 |
| TargetMol Chemicals Inc. | |
support@targetmol.com | United States | 38631 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 |
masar@topule.com | China | 8467 | 58 |
| Aladdin Scientific | |
tp@aladdinsci.com | United States | 52924 | 58 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131957 | 58 |
| Shanghai Biopharmaleader Co., Ltd. | +86 18721201413 |
sales@biopharmaleader.com | China | 1720 | 58 |
| Musechem | +1-800-259-7612 |
info@musechem.com | United States | 4660 | 60 |
| Fan De(Beijing) Biotechnology Co., Ltd. | 15911056312 |
liming@bio-fount.com | China | 9729 | 58 |
| Hangzhou Synstar pharmaceutical Technology CO.,Ltd | 0571-85361029 |
synstar518@163.com | China | 1990 | 58 |
- Beclabuvir
- Beclabuvir(BMS-791325)
- (1aR,12bS)-8-cyclohexyl-N-[(dimethylamino)sulfonyl]-1,1a,2,12b-tetrahydro-11-methoxy-1a-[(3-methyl-3,8-diazabicyclo[3.2.1]oct-8-yl)carbonyl]-cycloprop[d]indolo[2,1-a][2]benzazepine-5-carboxamide
- Cycloprop[d]indolo[2,1-a][2]benzazepine-9-carboxamide, 12-cyclohexyl-N-[(dimethylamino)sulfonyl]-4b,5,5a,6-tetrahydro-3-methoxy-5a-[(3-methyl-3,8-diazabicyclo[3.2.1]oct-8-yl)carbonyl]-, (4bS,5aR)-
- 958002-33-0
- C36H45N5O5S