(p-Chlorphenyl)phenylethandion Chemische Eigenschaften,Einsatz,Produktion Methoden
Synthese
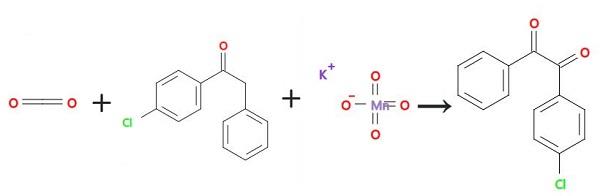
A benzene solution containing 51.2 g 4-chlorophenylacetic acid and 39.3 g thionyl chloride is refluxed for 6 hours and the solvent removed. The crude reaction product is added dropwise to a slurry of 40 g aluminum chloride in 200 ml benzene maintaining the temperature below 50° C. After addition the reaction is refluxed for 1.5 hours. After cooling, the mixture is poured into ice and the resulting white solid is separated yielding 61.3 g of crude product. The product is taken up in methylene chloride and washed with 5% sodium hydroxide solution followed by water, dried over sodium sulfate and stripped yielding 49.3 g (71%) of >95% pure 4'-chlorodeoxybenzoin. 15 g of 4-chlorodeoxybenzoin is dissolved in 105 ml pyridine. An aqueous solution containing 15 g potassium permanganate is added and the reaction stirred for 6 hours at room temperature using dry ice to buffer the pH near 7. The mixture is extracted with methylene chloride, the extract washed with water, dried over Na2SO4 and stripped. The residue is recrystallized from ethanol yielding 6.1 g of white plate-like crystals which is identified as starting material. Concentration of the mother liquor yields 4 g of 4-chlorobenzil.
(p-Chlorphenyl)phenylethandion Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

