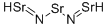Tristrontiumnitrid
|
|
Tristrontiumnitrid Eigenschaften
- Schmelzpunkt:
- 1030℃ [CIC73]
- L?slichkeit
- reacts with H2O; soluble in HCl
- Aggregatzustand
- Powder
- Farbe
- tan
- Sensitive
- moisture sensitive
- CAS Datenbank
- 12033-82-8
- EPA chemische Informationen
- Strontium nitride (Sr3N2) (12033-82-8)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Bildanzeige (GHS) |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | ||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||||||||||||||||
| Sicherheit |
|
Tristrontiumnitrid Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Strontium nitride should have the molecular formula of Sr3N2 and the molecular weight of 290.8752 g/mol. However, according to some sources, Strontium does not form the ionic compound Sr3N2 and Sr2N is the only compound that has been properly characterized to date. The reaction of Sr metal in a pure stream of nitrogen gas produces the “subnitride”:4Sr +N2 ? 2Sr2N
A second method would be the decomposition of an amide:
2Sr(NH2)2 + Heat ? 2Sr2N+ 2NH3 +H2
Physikalische Eigenschaften
In air, it strontium metal burns and produces strontium oxide and strontium nitride. But, since it does not react with nitrogen below 380 °C, it will only form the oxide spontaneously at room temperature. To produce the pure nitride, it is necessary to maintain the temperature above 380 °C in air or to heat the metal in pure nitrogen gas. In air, the molten mass, as it cools from 380 °C, tends to convert some of the nitride to oxide.Strontium nitride is described as Sr3N2,produced by burning strontium metal in air (resulting in a mixture with strontium oxide) or in nitrogen. Like other alkaline earth metal nitrides, it reacts with water to give strontium hydroxide and ammonia: Sr3N2 + 6H2O 3Sr(OH)2 + 2NH3
Verwenden
Strontium nitride (Sr3N2) can be used in the synthesis of ternary nitride oxide with molybdenum nitride under a low partial pressure of oxygen. It can also be used in the synthesis of barium-strontium silicate-based hollow structures for potential usage in drug delivery, catalysis, and sensors.Tristrontiumnitrid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Tristrontiumnitrid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 55)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29863 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 7732 | 58 |
| Henan Alfa Chemical Co., Ltd | |
China | 13179 | 58 | |
| Alfa Chemistry | |
Info@alfa-chemistry.com | United States | 24072 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 43340 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +86-18621343501; +undefined18621343501 |
product@acmec-e.com | China | 33338 | 58 |
| Aladdin Scientific | |
tp@aladdinsci.com | United States | 57505 | 58 |
12033-82-8(Tristrontiumnitrid)Verwandte Suche:
Praseodymnitrid
Yttriumnitrid
Cernitrid
Ytterbiumnitrid
Galliumnitrid
Lanthannitrid
Europiumnitrid
Tricalciumdinitrid
Aluminiumnitrid
Neodymnitrid
Chromnitrid
Titannitrid
Trilithiumnitrid
Trimagnesiumdinitrid
Dysprosiummononitrid
Erbiummononitrid
Strontium
Tristrontiumnitrid
- STRONTIUM NITRIDE
- tristrontium nitride
- Strontiumnitride99.5%
- StrontiuM nitride powder, -60 Mesh, 99.5%, Ba: <1%
- StrontiuM Nitride, Sr3N2, Powder
- StrontiuM Nitride, -60 Mesh
- StrontiuM nitride (99.5%-Sr)
- STRONTIUM NITRIDE ISO 9001:2015 REACH
- 12033-82-8
- Sr3N2
- N2Sr3