Allisartan Isoproxil Chemische Eigenschaften,Einsatz,Produktion Methoden
Clinical Use
Allisartan isoproxil, a member of a new class of selective angiotensin II-1 receptor antagonists, was
approved by the Chinese Food and Drug Administration (CFDA) for the treatment of hypertension in
July 2012.19 At time of publication, there is no trade name associated with this drug. Allisartan was
discovered and developed by the Chinese biomedical company Allist Pharmaceuticals. Allisartan
isoproxil is a prodrug which is readily hydrolyzed to active metabolite EXP3174, which is also the
active metabolite of losartan (des-triphenylmethyl-9).
Synthese
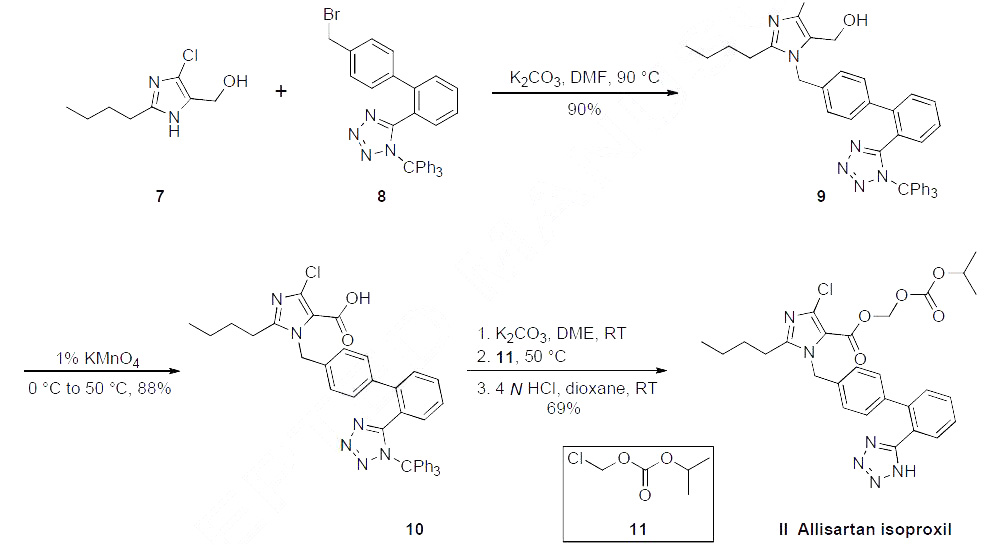
Although several synthetic routes
have been reported within two patents, the most likely scalable process route is described in the scheme. Commercial 2-butyl-4-chloro-5-(hydroxymethyl)-imidazole (7) was alkylated with Ntriphenylmethyl-
5-(4'-bromomethylbiphenyl-2-yl)tetrazole (8) under basic conditions in warm DMF,
providing alcohol 9 in 90% yield. This alcohol was then oxidized to the corresponding carboxylic acid
10 with KMnO4 in 88% yield. Etherification of acid 10 with isopropyl chloromethyl carbonate (11)
followed by de-tritylation of the tetrazole group under acidic contidions gave allisartan isoproxil (II) in
69% yield.
Allisartan Isoproxil Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

