Trimethylboroxine Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R11:Leichtentzündlich.
R34:Verursacht Ver?tzungen.
R41:Gefahr ernster Augensch?den.
R37/38:Reizt die Atmungsorgane und die Haut.
R19:Kann explosionsf?hige Peroxide bilden.
S-S?tze Betriebsanweisung:
S9:Beh?lter an einem gut gelüfteten Ort aufbewahren.
S16:Von Zündquellen fernhalten - Nicht rauchen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S33:Ma?nahmen gegen elektrostatische Aufladungen treffen.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S46:Bei Verschlucken sofort ?rztlichen Rat einholen und Verpackung oder Etikett vorzeigen.
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S3:Kühl aufbewahren.
Chemische Eigenschaften
colorless to yellow solution
Verwenden
Trimethylboroxine is used as a derivatizing agent for GLC analysis. It is used in a diverse array of areas, including as a polymerization additive. It is also used in the preparation of CBS catalysts for asymmetric reductions.
synthetische
Trimethylboroxine is prepared by heating B(CH3)3 and B2O3 together in a sealed tube. The B2O3 powder is made by dehydrating H3BO3 under vacuum over P2O5 , at 220℃. The very hygro-scopic oxide is placed with strict exclusion of moisture in a 200-ml. thick-wall Pyrex tube provided with a ground joint, and a melting-point capillary is fastened to the tube just below the joint. The tube is connected to a vacuum pump and immersed in liquid nitrogen, and when a high vacuum has been established, a quantity of B(CH3)3 equivalent to 4.25 g. (0.061 mole) of B2O3 is condensed in the tube. The tube is sealed off, heated to 600℃ and kept at this temperature for six hours; in the process the contents turn into a clear, colorless liquid. When the tube has cooled down, the tip is broken under a nitrogen blanket and sealed to a tube leading to the vacuum pump. The tube is evacuated and the contents of the tube are transferred into a -78℃ trap. The crude product is purified by removing volatile contaminants at -45℃ and then distilling the product from a -10℃ trap into a receiver held at -78℃.
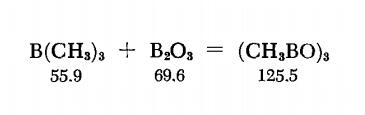
Application
Trimethylboroxine (TMB) is a cyclic anhydride of methyl-boronic acid. It can be been used:
As a derivatizing agent for gas chromatographic/mass spectrometric analysis.
In the preparation of methylaluminoxane (MAO) which is used in the polymerization of olefins.
As an electrolyte additive to enhance the interface stability of electrode/electrolyte.
In methylation of aryl halides by palladium-catalyzed Suzuki-Miyaura coupling reaction.
In the preparation of CBS (Corey, Bakshi and Shibata) catalysts for asymmetric reductions of ketones to alcohols.
l?uterung methode
Possible impurity is methylboronic acid. If present, then add a few drops of conc H2SO4 and distil it immediately, then fractionate it through an efficient column. [McCusker et al. J Am Chem Soc 79 5179 1957, IR: Goubeau & Keller Z Anorg Allgem Chem 272 303 1953, Beilstein 4 IV 4378.]
Trimethylboroxine Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

