Landiolol Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Landiolol was launched as iv infusion for the treatment of tachyarrhythmia
during surgery. This structurally related derivative of esmolol can be synthesized in 3 linear
steps from 3-(4-hydroxyphenyl)propionic acid by successive esterification followed by
alkylation of the phenol function with (2S)-glycidyltosylate and opening of the resulting
epoxide by the appropriate amine. Landiolol is an ultra short acting PI-adrenergic blocker
more cardioselective (βI/β2 = 255) than esmolol (βi/β2 = 32). It showed 6-8 times greater
efficiency compared to esmolol in reducing isoproterenol-induced increase in heart rate
and ventricular contraction in anesthetized dogs. In clinical trials, landiolol was effective
against a variety of arrhythmias with efficacy seen in patients with atrial fibrillation,
proxysmal supraventricular tachycardia, ventricular tachycardia and premature complexes.
Landiolol produced a doserelated pharmacokinetic behavior, has a rapid onset of action
(10 min.) and is rapidly hydrolyzed to inactive acidic metabolites by esterases after iv
administration. This results in an ultra-short half-life (approx. 3 min.) and p-blocade,
allowing rapid termination of the drug effect by termination of infusion if side effects occur.
Hypertension was the most frequent adverse event and resolved in less than 30 min. after
drug withdrawal.
Originator
Ono Pharmaceutical (Japan)
Trademarks
Onoact
Synthese
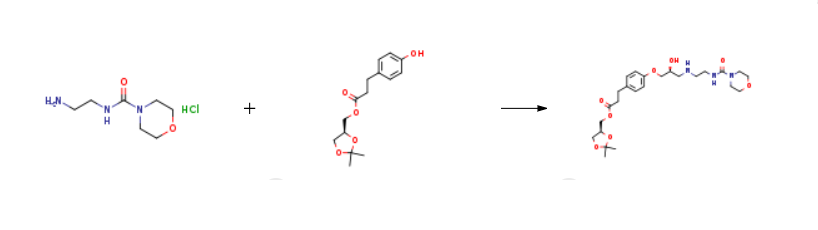
Landiolol is prepared by the reaction of 2-(morpholine-4-carboxamido)ethanamino hydrochloride and (S)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl 3-(4-hydroxyphenyl)propanoate. The steps are as follows:
A suspension of (S)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl 3-(4-((2R)-3-chloro-2-hydroxypropoxy)phenyl)propanoate prepared according Example 3 (0.50 g, 0.00134 mol) in isopropanol (10 ml) is added with 2-(morpholine-4-carboxamido)ethanamino hydrochloride (18) (1.4 g, 0.00670 mol), heated to 30-35°C and dropwise added with 30% NaOH, keeping pH at 10-11.
The mixture is left under stirring at 35-40°C, monitoring by UPLC.
After completion of the reaction, ethyl acetate (20 ml) and water (20 ml) are added and the phases are separated.
The organic phase is added with water (20 ml) and adjusted to pH 3-4 with hydrochloric acid.
The phases are separated and the resulting aqueous phase is then adjusted to pH 10-11 with sodium hydroxide and re-extracted with ethyl acetate (20 ml).
The solvent is then evaporated off under reduced pressure to obtain 0.38 g (55.6%) of a pale yellow oil which solidifies in time to a pale yellow solid.
Landiolol Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

