826-39-1
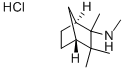 826-39-1 結(jié)構(gòu)式
826-39-1 結(jié)構(gòu)式
基本信息
鹽酸美加明
鹽酸美卡拉明
異坎胺
美加明
MECAMYLAMINE USP
inversine
MECAMYLAMINE
MECAMYLAMINE HYDROCHLORIDE
N,2,3,3-TETRAMETHYLBICYCLO[2.2.1]HEPTAN-2-AMINE HYDROCHLORIDE
3-methylaminoisocamphanehydrochloride
3-methylaminoisokamfanchlorid
inversinehydrochloride
mecaminehydrochloride
mekaminhydrochloride
mevasinhydrochloride
n,2,3,3-tetramethyl-2-norbornanaminehydrochloride
n,2,3,3-tetramethyl-2-norbornanaminhydrochloride
n-methyl-dl-isobornylaminehydrochloride
Mecamylamine HCl
MECAMYLAMINE USP
MecamycamineHydrochloride
Bicyclo2.2.1heptan-2-amine, N,2,3,3-tetramethyl-, hydrochloride
2-NORBORNANAMINE,N,2,3,3-TETRAMETHYL-,HYDROCHLORIDE
2-(Methylamino)isocamphane hydrochloride, Inversine, N,2,3,3-Tetramethylbicyclo[2.2.1]heptan-2-amine hydrochloride
物理化學(xué)性質(zhì)
安全數(shù)據(jù)
| 報價日期 | 產(chǎn)品編號 | 產(chǎn)品名稱 | CAS號 | 包裝 | 價格 |
| 2025/02/08 | HY-B1395 | 鹽酸美加明 Mecamylamine hydrochloride | 826-39-1 | 1 mg | 280元 |
| 2025/02/08 | HY-B1395 | 鹽酸美加明 Mecamylamine hydrochloride | 826-39-1 | 5mg | 700元 |
| 2025/02/08 | HY-B1395 | 鹽酸美加明 Mecamylamine hydrochloride | 826-39-1 | 10mM * 1mLin DMSO | 770元 |
常見問題列表
nAChR
Mecamylamine blocks muscle nAChRs in a use- and voltagedependent manner. Mecamylamine blocks the nicotinic currents via trapping mechanism. The main feature of this block is the phenomenon of block relief, which might be revealed by combined action of depolarization and activation of nAChRs. The experimental study of the Mecamylamine action on muscle nAChRs revealed that: (1) Mecamylamine (1-20 μM) reduces evoked end-plate currents (EPC) amplitude with Hill’s constant equal to 1.2 and IC 50 = 7.8 μM at holding potential –70 mV; (2) the calculated depth of its interaction with the muscle nAChR channel is almost half of the one of neuronal nAChRs (0.37 compare to 0.72 for neuronal nAChRs); (3) simultaneous membrane depolarization and repetitive activation of postsynaptic nAChRs by motor nerve stimulation produced rapid block relief dependent on the degree of depolarization, number of conditioning signals and Mecamylamine concentration, and only slightly depended on the rate of stimulation.
Mecamylamine (0.5-1 mg/kg; Intraperitoneal injection; C57BL/6J mice) has antidepressant-like effects in both the TST and FST and these effects are dependent on bothβ2 andα7 subunits.
| Animal Model: | C57BL/6J mice (3-4 months) forced swim test (FST) and tail suspension test (TST) |
| Dosage: | 0.5 mg/kg, 0.75 mg/kg, 1 mg/kg |
| Administration: | Intraperitoneal injection |
| Result: | Significantly decreased immobility time in the TST at the 1.0-mg/kg dose but did not alter baseline locomotor activity. Also significantly decreased time immobile in the FST. |
