184865-04-1
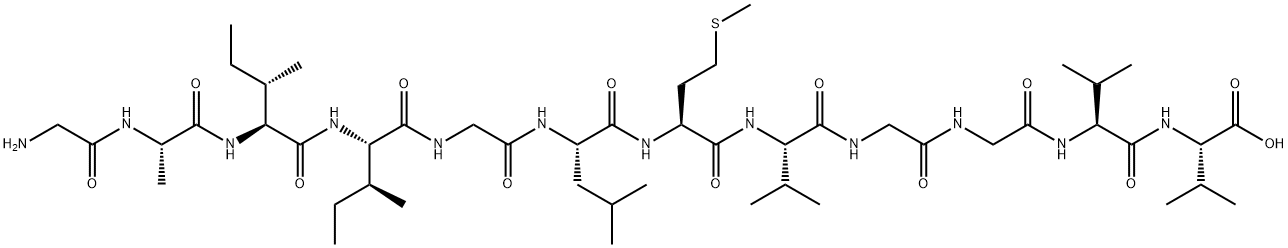 184865-04-1 結(jié)構(gòu)式
184865-04-1 結(jié)構(gòu)式
基本信息
AMYLOID Β-PROTEIN(30-40)
β-Amyloid 29-40
β-Amyloid (29-40)
Amyloid β-Protein
amyloidβ-protein(30-40)
Amyloid β-Protein (29-40)
AMyloid b-Protein (29-40)
物理化學(xué)性質(zhì)
| 報(bào)價(jià)日期 | 產(chǎn)品編號(hào) | 產(chǎn)品名稱 | CAS號(hào) | 包裝 | 價(jià)格 |
| 2023/03/20 | HY-P1522 | 淀粉Β-蛋白 β-Amyloid (29-40) | 184865-04-1 | 1mg | 800元 |
| 2023/03/20 | HY-P1522 | 淀粉Β-蛋白 β-Amyloid (29-40) | 184865-04-1 | 5mg | 2400元 |
| 2023/03/20 | HY-P1522 | 淀粉Β-蛋白 β-Amyloid (29-40) | 184865-04-1 | 10mg | 3900元 |
常見問(wèn)題列表
Amyloid-β
β-Amyloid (29-40) is a fragment of Amyloid-β peptide, unsoluble in water, acetonitrile or in a mixture of both in different ratios. Cationic arginine residues is introduced at β-Amyloid (29-40) C-terminus to solubilize β-Amyloid (29-40) and introduce cationicity in this Aβ stretch to enable it to interact with the negatively charged membrane of bacteria. β-Amyloid (29-40) variants shows antimicrobial effect. Single chain variable fragments (scFv's) binds the 17-28 region of Abeta and effectively inhibits in vitro aggregation of Abeta, but binding the carboxyl-terminal region of β-Amyloid (29-40) does not inhibit aggregation.
β-Amyloid Aggregation Guidelines (Following is our recommended protocol. This protocol only provides a guideline, and should be modified according to your specific needs).
1. Solid Aβ peptide was dissolved in cold hexafluoro-2-propanol (HFIP). The peptide was incubated at room temperature for at least 1h to establish monomerization and randomization of structure.
2. The HFIP was removed by evaporation, and the resulting peptide was stored as a film at -20 or -80 ℃.
3. The resulting film was dissolved in anhydrous DMSO at 5 mM and then diluted into the appropriate concentration and buffer (serum- and phenol red-free culture medium) with vortexing.
4. Next, the solution was age 48h at 4-8 ℃. The sample was then centrifuged at 14000g for 10 min at 4-8 ℃; the soluble oligomers were in the supernatant. The supernatant was diluted 10-200-fold for experiments.
Methods vary depends on the downstream applications.