| Identification | More | [Name]
4-METHOXYCINNAMIC ACID | [CAS]
943-89-5 | [Synonyms]
(2E)-3-(4-METHOXYPHENYL)ACRYLIC ACID
3-(4-METHOXY-PHENYL)-ACRYLIC ACID
4-METHOXYCINNAMIC ACID
AKOS B000181
AKOS BB/0034
AKOS BBS-00000769
(E)-3-(4-METHOXYPHENYL)ACRYLIC ACID
LABOTEST-BB LT00108121
METHOXYCINNAMIC ACID,4-
O-METHYL-P-COUMARIC ACID
O-METHYL-P-CUMARIC ACID
OTAVA-BB BB0123400502
PMCA
P-METHOXYCINNAMIC ACID
RARECHEM BK HC T257
TIMTEC-BB SBB005717
TRANS-3-(4-METHOXYPHENYL)ACRYLIC ACID
TRANS-4-METHOXYCINNAMIC ACID
(E)-p-methoxycinnamic acid
2-Propenoic acid, 3-(4-methoxyphenyl)-, (2E)- | [EINECS(EC#)]
213-405-4 | [Molecular Formula]
C10H10O3 | [MDL Number]
MFCD00004398 | [Molecular Weight]
178.18 | [MOL File]
943-89-5.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [RTECS ]
UD3391300
| [F ]
8 | [TSCA ]
Yes | [HS Code ]
2918.29.7500 | [HazardClass ]
IRRITANT |
| Hazard Information | Back Directory | [Uses]
Esters derived from trans-4-methoxycinnamic acid are effective absorbers of UV radiation. The starting material was trans-4-methoxycinnamic acid and 3,5dimethoxybenzoic acid methyl ester in isolated avenanthramide alkaloids synthsis. trans-4-Methoxycinnamic acid was converted into its acid chloride (yield 98%) with thionyl chloride. Employed in organic synthesis of pharmaceutical intermediates, synthetic anti-adrenergic drugs esmolol, cosmetics ultraviolet absorption. | [Definition]
ChEBI: 4-methoxycinnamic acid is a methoxycinnamic acid having a single methoxy substituent at the 4-position on the phenyl ring. It is functionally related to a cinnamic acid. | [Preparation]
4-methoxycinnamic acid synthesis : In a 25-mL, roundbottomed flask, 4-methoxybenzaldehyde (0.804 mL, 6.61 mmol) (3), malonic acid (1.75 g, 16.8 mmol), and β-alanine (0.10 g, 1.12 mmol) were dissolved in pyridine (3.0 mL, 37.1 mmol) and heated under reflux for 90 minutes. After cooling to room temperature, the reaction mixture was placed in an ice bath and 8.0 mL of concentrated HCl was slowly added. The resulting white precipitate was collected by vacuum filtration, washed with cold water (2 x 10mL) and dried thoroughly. The product was recrystallized from absolute ethanol (~20 mL).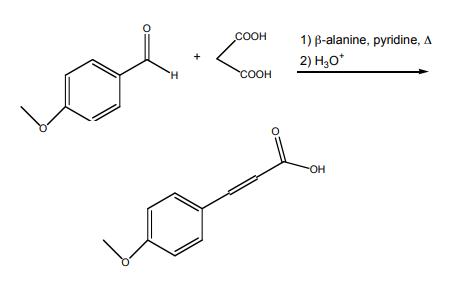
Synthesis of 4-methoxycinnamic acid | [Purification Methods]
Crystallise the acid from MeOH to constant melting point and UV spectrum. [Beilstein 10 IV 1005.] |
|
|