| Identification | More | [Name]
CHALCONE | [CAS]
94-41-7 | [Synonyms]
1,3-DIPHENYL-2-PROPEN-1-ONE
1,3-DIPHENYL-2-PROPEN-I-ONE
1,3-DIPHENYL-2-PROPENONE
1,3-DIPHENYL-3-PROPEN-1-ONE
1,3-DIPHENYLPROP-2-EN-1-ONE
2-BENZALACETOPHENONE
2-BENZYLIDENEACETOPHENONE
(2E)-1,3-DIPHENYLPROP-2-EN-1-ONE
3-PHENYLACRYLOPHENONE
BENZALACETOPHENONE
BENZYLIDENEACETOPHENONE
BENZYLIDINEACETOPHENONE
CHALCONE
CHALKONE
(E)-CHALCONE
PHENYL STYRYL KETONE
TRANS-BENZYLIDENEACETOPHENONE
TRANS-CHALCONE
(2E)-1,3-Diphenyl-2-propen-1-one
1,3-Diphenyl-1-propen-3-one | [EINECS(EC#)]
202-330-2 | [Molecular Formula]
C15H12O | [MDL Number]
MFCD00003082 | [Molecular Weight]
208.26 | [MOL File]
94-41-7.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R36/37:Irritating to eyes and respiratory system . | [Safety Statements ]
S22:Do not breathe dust .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [WGK Germany ]
3
| [RTECS ]
UD5576750
| [HS Code ]
29143900 | [Safety Profile]
Poison by intravenous
route. See also KETONES. When heated to
decomposition it emits acrid smoke and
irritating fumes. |
| Hazard Information | Back Directory | [Description]
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids.They show antibacterial, antifungal, antitumor and anti-inflammatory properties. They are also intermediates in the biosynthesis of flavonoids, which are substances widespread in plants and with an array of biological activities. Chalcones are also intermediates in the Auwers synthesis of flavones. | [Chemical Properties]
light yellow powder | [Uses]
1,3-Diphenyl-2-propenone was used in the preparation of pharmacologically-interesting heterocyclic systems like pyrazolines and pyrimidines. | [Uses]
Chalcone is used in the preparation of pharmacologically-interesting heterocyclic systems like pyrazolines and pyrimidines. It also inhibits the proliferation of human breast cancer cell lines, MCF-7 and MDA-MB-231 by inducing apoptosis and blocking cell cycle progression in the G2/M phase. | [Definition]
ChEBI: A member of the class of chalcones that is acetophenone in which one of the methyl hydrogens has been replaced by a benzylidene group. | [Preparation]
Chalcone is an aromatic ketone that forms the central core for a variety of important biological compounds, which are known collectively as chalcones.
Chalcones can be prepared by an aldol condensation between a benzaldehyde and an acetophenone in the presence of sodium hydroxide as a catalyst.
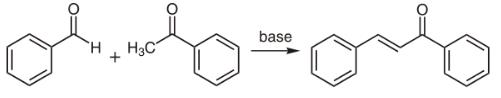
This reaction has been found to work without any solvent at all - a solid-state reaction. The reaction between substituted benzaldehydes and acetophenones has been used to demonstrate green chemistry in undergraduate chemistry education.In a study investigating green chemistry synthesis, chalcones were also synthesized from the same starting materials in high temperature water (200 to 350 °C). | [Biological Activity]
1,3-Diphenyl-2-propenone (chalcone) inhibits the proliferation of human breast cancer cell lines, MCF-7 and MDA-MB-231 by inducing apoptosis and blocking cell cycle progression in the G2/M phase. It is an inhibitor of Plasmodium falciparum cyclin-dependent protein kinases. | [Biochem/physiol Actions]
1,3-Diphenyl-2-propenone (chalcone) inhibits the proliferation of human breast cancer cell lines, MCF-7 and MDA-MB-231 by inducing apoptosis and blocking cell cycle progression in the G2/M phase. It is an inhibitor of Plasmodium falciparum cyclin-dependent protein kinases. | [storage]
Store at -20°C | [Purification Methods]
Crystallise it from EtOH by warming to 50o (about 5mL/g), iso-octane, or toluene/pet ether, or recrystallise it from MeOH, and then twice from hexane. SKIN IRRITANT. [Beilstein 7 IV 1658.] |
|
|