| Identification | More | [Name]
3-Chloroperoxybenzoic acid | [CAS]
937-14-4 | [Synonyms]
3-CHLOROPERBENZOIC ACID
3-CHLOROPEROXYBENZOIC ACID
3-Chloroperoxy benzoic acid(more than 57% but not more than 86%,with 3-chorobenzoic acid)
M-CHLORO BENZOYL HYDROPEROXIDE
M-CHLOROPERBENZOIC ACID
M-CHLOROPEROXYBENZOIC ACID
M-CPBA
3-Chlorobenzenecarboperoxoic acid
3-chloro-benzenecarboperoxoicaci
3-chlorobenzenecarboperoxoicacid
Benzenecarboperoxoic acid, 3-chloro-
m-chloro-peroxybenzoicaci
Perbenzoic acid, m-chloro-
Peroxybenzoic acid, m-chloro-
3-Choroperoxybenzoic acid
m-choroperoxybenzoic acid
3-CHLOROPEROXYBENZOIC ACID, 77% MAX.
metaChloro Perbenzoic Acid
3-Chloroperoxybenzoic acid, tech., wet with water, 50-55%
3-Chloroperoxybenzoicacid,tech.70-77%,wetwithwater | [EINECS(EC#)]
213-322-3 | [Molecular Formula]
C7H5ClO3 | [MDL Number]
MFCD00002127 | [Molecular Weight]
172.57 | [MOL File]
937-14-4.mol |
| Chemical Properties | Back Directory | [Appearance]
white powder | [Melting point ]
69-71 °C(lit.)
| [Boiling point ]
244.67°C (rough estimate) | [density ]
0.56
| [vapor pressure ]
0.373Pa at 25℃ | [refractive index ]
1.4580 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) | [form ]
Moist Powder | [pka]
7.57 (in water @ 25 °C) | [color ]
White | [Odor]
slight pungent odor | [PH]
4.5@25 °C (saturated aq. sol) | [Stability:]
Strong oxidizing agent-contact with combustible material may cause fire. May be shock or heat sensitive. Incompatible with organic materials, strong reducing agents. | [Water Solubility ]
insoluble | [Decomposition ]
>88 °C | [Detection Methods]
HPLC | [BRN ]
608317 | [InChIKey]
NHQDETIJWKXCTC-UHFFFAOYSA-N | [LogP]
1.03 at 25℃ | [CAS DataBase Reference]
937-14-4(CAS DataBase Reference) | [NIST Chemistry Reference]
3-Chloroperbenzoic acid(937-14-4) | [EPA Substance Registry System]
937-14-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
O,Xi,C | [Risk Statements ]
R5:Heating may cause an explosion.
R8:Contact with combustible material may cause fire.
R36/37/38:Irritating to eyes, respiratory system and skin .
R43:May cause sensitization by skin contact.
R7:May cause fire.
R34:Causes burns.
R22:Harmful if swallowed. | [Safety Statements ]
S17:Keep away from combustible material .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S3/7:Keep container tightly closed in a cool place .
S14:Keep away from ... (incompatible materials to be indicated by the manufacturer) .
S27:Take off immediately all contaminated clothing .
S7/9:Keep container tightly closed and in a well-ventilated place . | [RIDADR ]
UN 3106 5.2
| [WGK Germany ]
3
| [RTECS ]
SD9470000
| [F ]
4.4 | [TSCA ]
Yes | [HazardClass ]
5.2 | [PackingGroup ]
II | [HS Code ]
29163990 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
1,4-Dioxane-->Magnesium sulfate heptahydrate-->Polyethylene-->3-Chlorobenzoyl chloride | [Preparation Products]
3,5-DIMETHYLPYRIDIN-4-AMINE-->2-(2,3-DIHYDRO-1-BENZENESULFONYL-PYRROLO[2,3-B]PYRIDIN-3-YL)ACETONITRILE-->4,5-DICHLORO-2-METHYLPYRIDINE-->METHYL(2R,3S)-2,2-DIMETHYL-3-(2-OXOPROPYL)-CYCLOPROPANEACETATE-->2,5-Dichloropyridine-->1-PROPANESULPHONYLACETONITRILE-->5-Bromo-2-hydroxymethylpyridine-->5-Bromopyridine-2-carbaldehyde-->3-AMINOMETHYL-PYRIDINE-2-CARBOXYLIC ACID-->4-(CHLOROSULFONYL)-7-FLUORO-2,1,3-BENZOXADIAZOLE-->4-Chloro-2-(methylsulfonyl)pyrimidine-->S-METHYL-S-(2-METHYLPYRAZINYL) SULFOXIMINE-->4-[(TERT-BUTOXYCARBONYLAMINO)METHYL]-2-CYANOPYRIDINE-->4-(BOC-AMINOMETHYL)PYRIDINE-2-CARBOXYLIC ACID-->4-FLUORO-2,1,3-BENZOXADIAZOLE-->LORACARBEF (200 MG)-->1,4-OXATHIANE SULFOXIMINE-->3-(TERT-BUTOXYCARBONYLAMINO-METHYL)-PYRIDINE-2-CARBOXYLIC ACID-->Omeprazole-->Tacalcitol-->8-HYDROXYQUINOLINE-2-CARBONITRILE-->5-BROMO-4-CHLORO-2-METHANESULFONYL-PYRIMIDINE-->1,2-Epoxyhexane-->1,7-DIDEAZAADENINE-->1H-PYRROLO[2,3-B]PYRIDINE, 4-NITRO--->5-BROMO-2-METHYLPYRIDINE N-OXIDE-->4-Bromo-7-azaindole-->NITROCYCLOHEXANE-->1,2-EPOXY-9-DECENE-->6-BROMO-1H-PYRROLO[2,3-B]PYRIDINE-->6-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE-->3-Isochromanone-->4-(METHYLSULFINYL)PHENOL-->1H-Pyrrolo[2,3-b]pyridine, 4-nitro-, 7-oxide-->4-IODO-7-AZAINDOLE-->4-Chloro-7-azaindole-->Cyclopentene oxide-->Pantoprazole-->3-NITROSOBENZAMIDE-->7-OXIDE-7-AZAINDOLE |
| Hazard Information | Back Directory | [General Description]
This solid peroxide is sensitive to heat. Storage of this material must be done so with stringent temperature control measures. It's explosion hazard is also mitigated by mixing the peroxide in a solvent slurry. | [Reactivity Profile]
May explode from heat, shock, friction or contamination. May ignite combustibles (wood, paper, oil, clothing, etc.). May be ignited by heat, sparks or flames. | [Definition]
ChEBI: 3-chloroperbenzoic acid is a peroxy acid and a member of monochlorobenzenes. | [Reactions]
3-Chloroperoxybenzoic acid (MCPBA) is one of the most popular oxidation reagent in organic synthesis, because of its outstanding performance in terms of:
reactivity, combined with reducing the number of reaction steps in classical synthetic routes,
regio- and stereoselectivity,
protection of functional groups mostly not required,
high purity and yields.
Its literature covers a huge area of different syntheses and below reaction equations just can be a brief overview of its interesting applications:
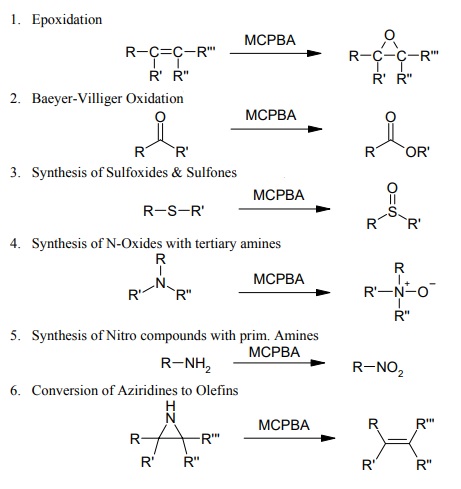 | [Synthesis Reference(s)]
Synthetic Communications, 19, p. 1271, 1989 DOI: 10.1080/00397918908054534 | [reaction suitability]
reagent type: oxidant | [Purification Methods]
Recrystallise MCPBA from CH2Cl2 [Traylor & Mikztal J Am Chem Soc 109 2770 1987]. Peracid of 99+% purity can be obtained by washing commercial 85% material with phosphate buffer pH 7.5 and drying the residue under reduced pressure. Alternatively the peracid can be freed from m-chlorobenzoic acid by dissolving 50g/L of *benzene and washing with an aqueous solution buffered at pH 7.4 (NaH2PO4/NaOH) (5 x 100mL). The organic layer is dried over MgSO4 and carefully evaporated under vacuum. Necessary care should be taken in case of EXPLOSION. The solid is recrystallised twice from CH2Cl2/Et2O and stored at 0o in a plastic container as glass catalyses the decomposition of the peracid. The acid is assayed iodometrically. [Schwartz & Blumbrgs J Org Chem 29 1976 1964, Bortolini et al. J Org Chem 52 5093 1987, McDonald et al. Org Synth Coll Vol VI 276 1988, Beilstein 9 IV 972.] |
| Questions And Answer | Back Directory | [Chemical Properties]
3-chloroperoxybenzoic acid is white powdery crystals. Melting point is 92-94°C (decomposed). It is almost insoluble in water, but soluble in ethanol, ethers, chloroform, and dichloroethane. It is thermally stable and has an annual decomposition rate of less than 1% at room temperature. The decomposition rate is accelerated in the liquid state. 3-Chloroperbenzoic acid is sensitive to heat and shock, and pure solid. 3-Chloroperbenzoic acid is flammable and potentially explosive. It contains a weak –O–O– bond and a nucleophilic OH group, that makes it versatile oxidative and easily breakable. | [Uses]
3-Chloroperoxybenzoic acid is commonly used in double bond epoxidation, nitridation, cyclization, Baeyer-Villiger oxidation, and N-oxidation. It can also be used as an oxidant for fine chemicals such as synthetic medicine and pesticides. It is also sometimes used as a bleaching agent [1-6].
• Used in cyclization reaction, Baeyer-Villiger reaction, N-oxidation reaction and S-oxidation reaction.
• Used as an oxidant for fine chemical products such as synthetic medicine and pesticides.
• Used as oxidant and bleach.
• As a good electrophilic reagent, it can react with many functional groups and can oxidize olefins, enol silyl ethers, furans, sulfides, selenides and amino compounds.
|
|
|