| Identification | Back Directory | [Name]
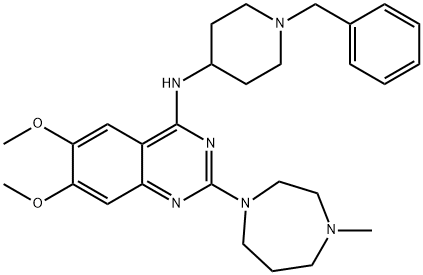
BIX 01294, 2-(Hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-6,7-dimethoxy-N-[1-(phenylmethyl)-4-piperidinyl]-4-quinazolinamine hydrate trihydrochloride | [CAS]
935693-62-2 | [Synonyms]
CS-207
BIX 01294
BIX01294,BIX-01294
BIX 01294 (free base)
BIX-01294;BIX 01294; BIX01294
BIX 01294 hydrate trihydrochloride
Histone Lysine methyltransferase Inhibitor
Histone Lysine Methyltransferase Inhibitor - CAS 935693-62-2 - Calbiochem
N-(1-Benzyl-4-piperidinyl)-6,7-dimethoxy-2-(4-methyl-1,4-diazepan-1-yl)-4-quinazolinamine
N-(1-Benzylpiperidin-4-yl)-6,7-dimethoxy-2-(4-methyl-1,4-diazepan-1-yl)quinazolin-4-amine trih
2-(Hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-6,7-dimethoxy-N-[1-(phenylmethyl)-4-piperidinyl]-4-quina
2-(Hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-6,7-dimethoxy-N-[1-(phenylmethyl)-4-piperidinyl]-4-quinazolinamine
4-QuinazolinaMine, 2-(hexahydro-4-Methyl-1H-1,4-diazepin-1-yl)-6,7-diMethoxy-N-[1-(phenylMethyl)-4-piperidinyl]-
2-(Hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-6,7-dimethoxy-N-[1-(phenylmethyl)-4-piperidinyl]-4-quinazolinaminetrihydrochloride
2-(Hexahydro-4-Methyl-1H-1,4-diazepin-1-yl)-6,7-diMethoxy-N-[1-(phenylMethyl)-4-piperidinyl]-4-quinazolinaMine hydrate trihydrochloride
BIX 01294, 2-(Hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-6,7-dimethoxy-N-[1-(phenylmethyl)-4-piperidinyl]-4-quinazolinamine hydrate trihydrochloride | [Molecular Formula]
C28H38N6O2 | [MDL Number]
MFCD11045283 | [MOL File]
935693-62-2.mol | [Molecular Weight]
490.64 |
| Chemical Properties | Back Directory | [Boiling point ]
654.6±65.0 °C(Predicted) | [density ]
1.195±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
Soluble in DMSO (up to 50 mg/ml), or in Water (up to 50 mg/ml). | [form ]
Off-white solid | [pka]
9.34±0.70(Predicted) | [color ]
Off-white | [Water Solubility ]
H2O: >20mg/mL | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 3 months. | [InChIKey]
BZOWJAYUMMHCDW-UHFFFAOYSA-N |
| Hazard Information | Back Directory | [Description]
BIX-01294 (935693-62-2) is a selective inhibitor of G9a histone methyltransferase (G9aHMTase; IC50 = 1.7 μM) as well as GLP HMTase (IC50 = 38 μM) leading to a decrease in H3K9me2(histone H3 lysine 9 methylation) in vitro.1 BIX-01294 facilitates the reactivation of pluripotency genes and induces passive demethylation, thus promoting reprogramming. BIX-01294, in combination with BAY K8644 (a calcium channel agonist), was found to improve reprogramming efficiencies of Oct4-Klf4-(OK)-infected neural progenitor cells.3 | [Uses]
BIX 01294 is a euchromatic histone-lysine N-methyltransferase 2 (EHMT2) inhibitor, induces autophagy and apoptosis in human neuroblastoma cells, specifically human bladder cancer cells. | [Definition]
ChEBI: 6,7-dimethoxy-2-(4-methyl-1,4-diazepan-1-yl)-N-[1-(phenylmethyl)-4-piperidinyl]-4-quinazolinamine is a member of piperidines. | [General Description]
A cell-permeable diazepinyl-quinazolinamine, non-SAM (S-adenosylmethionine) analog-based HMTase (histone methyltransferase) inhibitor that selectively interferes with the G9a-catalyzed H3K9me2 (histone H3 Lys9 dimethylation) modification (IC50 = 1.7 μM) in a reversible manner. It inhibits the GLP-catalyzed H3K9me3 only at much higher concentrartions (IC50 = 38 μM) and exhibits little activity against H3 methylations catalyzed by other HMTases (PRMT1, SET7/9, ESET, SUV39H1). Shown to effectively synergize with Oct3/4 and Klf4 in inducing reprogramming of primary murine fetal NPCs (Neural Progenitor Cells) into iPS (induced Pluripotent Stem) cells without additional viral transduction of Sox2 and c-Myc. | [Biological Activity]
G9a-like protein and G9a histone lysine methyltransferase (HMTase) inhibitor (IC 50 values are 0.7 and 1.7 μ M respectively) that displays no activity at other HTMases up to 37 μ M. Modulates H3K9me2 levels in mammalian cells and potentiates induction of pluripotent stem cells from somatic cells in vitro . | [target]
G9a-like protein | [References]
1) Kubicek et al. (2007) Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase; Mol. Cell. 25 473
2) Huangfu et al. (2008) Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds; Nat. Biotechnol. 26 795
3) Shi et al., (2008) A combined chemical and genetic approach for the generation of induced pluripotent stem cells; Cell Stem Cell. 2 525 |
|
|