| Identification | Back Directory | [Name]
2-(tert-butoxycarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid | [CAS]
851784-82-2 | [Synonyms]
Lifitegrast intermediate
-(Tert-Butoxycarbonyl-5,7-Dichloro-1,2,3,4-T..
2-Boc-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic Acid
N-Boc- 5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid
2-(tert-butoxycarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid
5,7-Dichloro-3,4-dihydro-2,6(1H)-isoquinolinedicarboxylic acid 2-(1,1-dimethylethyl) ester
2,6(1H)-Isoquinolinedicarboxylic acid, 5,7-dichloro-3,4-dihydro-, 2-(1,1-dimethylethyl) ester
5,7-dichloro-2-[(2-methylpropan-2-yl)oxycarbonyl]-3,4-dihydro-1H-isoquinoline-6-carboxylic acid | [Molecular Formula]
C15H17Cl2NO4 | [MDL Number]
MFCD22370640 | [MOL File]
851784-82-2.mol | [Molecular Weight]
346.21 |
| Chemical Properties | Back Directory | [Boiling point ]
468.5±45.0 °C(Predicted) | [density ]
1.379±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Sparingly, Sonicated), Methanol (Slightly) | [form ]
Solid | [pka]
1.87±0.20(Predicted) | [color ]
White to Off-White | [InChI]
InChI=1S/C15H17Cl2NO4/c1-15(2,3)22-14(21)18-5-4-9-8(7-18)6-10(16)11(12(9)17)13(19)20/h6H,4-5,7H2,1-3H3,(H,19,20) | [InChIKey]
JXBQRXIJBFUZMR-UHFFFAOYSA-N | [SMILES]
C1C2=C(C(Cl)=C(C(O)=O)C(Cl)=C2)CCN1C(OC(C)(C)C)=O |
| Hazard Information | Back Directory | [Description]
2-(tert-Butoxycarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic Acid is a carboxylic acid derivative of tetrahydroisoquinoline with a Boc group and two chlorine atoms attached to the tetrahydroisoquinoline ring. It may exhibit various biological activities, such as anticancer, anti-inflammatory, and anticonvulsant properties, depending on the nature and position of the substituents.
| [Uses]
2-(tert-Butoxycarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic Acid is a reagent in the preparation of N-benzofuranylcarbonyl dichlorotetrahydroisoquinolinylcarbonylmethylsulfonyl L-phenylalanine as LFA-1 inhibitor and polymorph thereof for the treatment of LFA -1 mediated diseases. | [Synthesis]
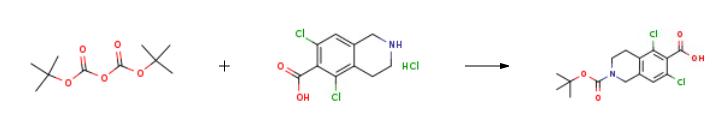
Boc-protection was used for the ring nitrogen in the intermediates 21 and 22.Compound 5 was deprotected with HC1 in dioxane to produce compound 23 in better than 97%yield. Boc-protection was introduced, using di-tert-butyl dicarbonate (1 .1 equivalent), and compound 21 was obtained in better than 95% yield. Compound 10 was coupled with compound 21 to obtain compound 22, using HATU and triethylamine in DMF. The product, compound 22, was obtained in quantitative yield, and greater than 90% purity. Deprotection with HC1 yielded the compound of Formula 12 in 97.4% yield. |
|
|