| Identification | Back Directory | [Name]
TMC-207 | [CAS]
843663-66-1 | [Synonyms]
TMC-207
Sirturo
R 207910
Bedaquiline
bedaquinline
Bedaquiline D6
(αS,βR)-Bedaquiline
Bedaquiline (TMC-207)
Bedaquiline(R 207910,TMC 207)
1-(6-Bromo-2-methoxy-quinolin-3-yl)-4-dimethylamino-2-naphthalen-1-yl-1-phenyl-butan-2-ol
(1R)-(6-Bromo-2-methoxyquinolin-3-yl)-4-(dimethylamino)-2(S)-(1-naphthyl)-1-phenylbutan-2-ol
(αS,βR)-6-Bromo-α-[2-(dimethylamino)ethyl]-2-methoxy-α-1-naphthalenyl-β-phenyl-3-quinolineethanol
(1R,2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-4-(methylamino)-2-(naphthalen-1-yl)-1-phenylbutan-2-ol
(1R,2S)-1-(6-BroMo-2-Methoxyquinolin-3-yl)-4-(diMethylaMino)-2-(naphthalen-1-yl)-1-phenylbutan-2-ol
6-Bromo-alpha-[2-(dimethylamino)ethyl]-2-methoxy-alpha-1-naphthalenyl-beta-phenyl-3-quinolineethanol
3-Quinolineethanol, 6-bromo-α-[2-(dimethylamino)ethyl]-2-methoxy-α-1-naphthalenyl-β-phenyl-, (αS,βR)-
3-Quinolineethanol, 6-bromo-alpha-(2-(dimethylamino)ethyl)-2-methoxy-alpha-1-naphthalenyl-beta-phenyl-, (alphas,betar)- | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C31H29BrN2O | [MOL File]
843663-66-1.mol | [Molecular Weight]
525.487 |
| Chemical Properties | Back Directory | [Melting point ]
104 °C | [Boiling point ]
702.7±60.0 °C(Predicted) | [density ]
1.322±0.06 g/cm3(Predicted) | [storage temp. ]
Room temperature | [solubility ]
Soluble in DMSO (10 mg/ml) | [form ]
solid | [pka]
13.05±0.29(Predicted) | [color ]
White | [optical activity]
[α]/D 195.0 to 155.0° (c = 0.5g/100mL in DMF) | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. | [CAS DataBase Reference]
843663-66-1 |
| Hazard Information | Back Directory | [Uses]
(αS,βR)-Bedaquiline is a diarylquinoline derivative that acts as a mycobacterial inhibitor. Bedaquiline shows promise as potential drug in the treatment of tuberculosis. | [Uses]
Labeled Bedaquiline, intended for use as an internal standard for the quantification of Bedaquiline by GC- or LC-mass spectrometry. | [Description]
In December 2012, the US FDA approved bedaquiline as part of combination
therapy for the treatment of multi-drug resistant tuberculosis (MDRTB).
Bedaquiline is the first drug approved for MDR-TB and is the first
approval from a new class of antituberculosis agents in the past 40 years.
Due to the high unmetmedical need for treating MDR-TB, the FDA granted
bedaquiline accelerated approval based on Phase II results, providing patients access to the drug while additional clinical studies are carried out. Bedaquiline (also known as TMC207 and R207910) is a diarylquinoline
that was discovered from a high-throughput, whole-cell screening
strategy with Mycobacterium smegmatis used as a surrogate for
M. tuberculosis. Bedaquiline is a single enantiomer of an initial screening
hit. Bedaquiline has potent and selective activity against mycobacteria, and
is active against both drug-sensitive and drug-resistant M. tuberculosis. The mechanism of action of bedaquiline is unique amongst anti-TB drugs and
involves inhibition of mycobacterial ATP synthase; it is not active against
human ATP synthase. Bedaquiline has in vivo activity in numerous preclinical models of TB infection, alone and in combination with other anti-TB agents, and has bactericidal activity in established TB infection models. Bedaquiline is synthesized in five steps from 3-phenylpropionic acid
and para-bromoaniline. Following amide formation, treatment with POCl3
and DMF under Vilsmeier–Hack conditions gave a 2-chloroquinoline product.
Treatment with sodium methoxide, followed by condensation with
3-(dimethylamino)-1-(naphthalen-1-yl)propan-1-one, and separation of isomers
gave bedaquiline. | [Description]
TMC207 is a diarylquinoline that selectively inhibits the proton pump of the mycobacterial ATP synthase.1 It demonstrates potent activity against both drug-sensitive and drug-resistant M. tuberculosis and other mycobacterial species with MIC50 values of ~0.03 μg/ml.1 | [Originator]
Janssen (Belgium) | [Definition]
ChEBI: Bedaquiline is a quinoline-based antimycobacterial drug used (as its fumarate salt) for the treatment of pulmonary multi-drug resistant tuberculosis by inhibition of ATP synthase, an enzyme essential for the replication of the mycobacteria. It has a role as an antitubercular agent and an ATP synthase inhibitor. It is a member of quinolines, a member of naphthalenes, an organobromine compound, an aromatic ether, a tertiary alcohol and a tertiary amino compound. It is a conjugate base of a bedaquiline(2+). | [Brand name]
Sirturo | [storage]
Store at -20°C | [References]
Andries et al. (2005), A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis; Science, 307 223
Koul et al. (2007), Diarylquinolines target subunit c of mycobacterial ATP synthase; Nat. Chem. Biol., 3 323
Biukovic et al. (2013), Variations of subunit {varepsilon} of the Mycobacterium tuberculosis F1F0 ATP synthase and a novel model for mechanism of action of the tuberculosis drug TMC207; Antimicrob. Agents Chemother., 57 168
Sarathy et al. (2019), Re-Understanding the Mechanisms of Action of the Anti-Mycobacterial Drug Bedaquiline; Antibiotics (Basel), 8 261
Ghahremanpour et al. (2020), Identification of 14 Known Drugs as Inhibitors of the Main Protease of SARS-CoV-2; ACS Med. Chem. Lett., 11 2526 |
| Questions And Answer | Back Directory | [Overview]
Bedaquiline, formally called (1R, 2S)-1-(6-Bromo-2-methoxy-3-quinolinyl)-4-(dimethylamino)-2-(1-naphthyl)-1-phenyl-2-butanol in chemistry and known as Sirturo in commercial, is a new anti-mycobacterial medicine of diarylquinolines. It impinges on the
ATP synthesis of Mycobacterium tuberculosis by inhibiting the activity of proton pump on the cell’s ATP synthetase, and thereby eliminates M. tuberculosis (TB). It’s used for adult multi-drug resistant tuberculosis (MDR-PTB). | [Synthetic Methods]
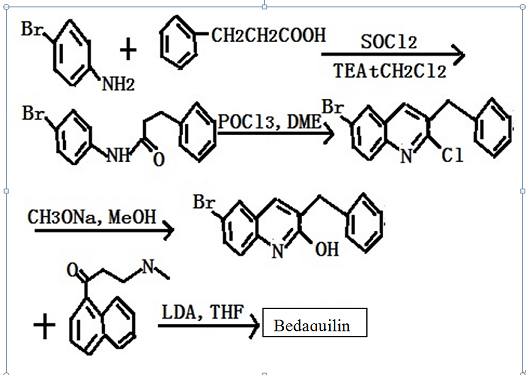
Fig: Synthetic route of Bedaquiline | [Pharmacological Function]
Bedaquinoline has the same bactericidal activity against both sensitive and resistant strains of mycobacterium tuberculosis as well as on dormant bacteria. | [Pharmacology]
The proton pump on the cell’s ATP synthetase, which is a crucial enzyme for M. tuberculosis (TB) to synthesize ATP, is the unique and specific locus of Bedaquinoline.
After the combination onto the oligomer and lipoprotein subunit c, Bedaquinoline can inhibit the synthesis of ATP and bring the death to the bacterium cell. Compared with those existing anti-TB medicines, it presents a novel pharmacology and no cross resistance effect was found between Bedaquinoline and other anti-TB medicines. The gene sequence of the subunit c of ATP synthetase is named as atpE, whose amino acid sequence is highly conservative. The resistance of TB to Bedaquinoline comes from its reduced combination onto subunit c of ATP synthetase due to the mutation of the 63rd or 66th on atpE. | [Pharmacokinetics]
Bedaquinoline is easy for oral assimilation. The bioavailability of Bedaquinoline taken with food is twice higher than when taken with an empty stomach. It reaches its blood concentration in 5 hours after taken and has a plasma protein binding rate of 99.9% as well as a plasma half-life of 173 hours. Bedaquinoline can be widely distributed in human body with a homeostasis distribution volume of 1000L. Its clearance rate is low enough and the elimination half-life is 5.5 months. Bedaquinoline is metabolize into metabolite 1~8 in the demethylation mainly through CYP3A4 and partly though CYP2C8 and CYP2C19. Metabolite 2 (M2), the most important metabolite, which has only 1/3-1/6 the activity of Bedaquinoline, yet present a more strong cytotoxicity and is more likely to cause the drug-induced phospholipidosis. Bedaquinoline and its metabolites are mostly excreted by feces, only 1% to 4% by urine. | [Adverse Effect]
Common adverse reactions are nausea, headache, arthralgia, loss of appetite, vomiting and rash, dizziness, elevated transaminase, increased hemodiastase, muscle pain, diarrhea and prolonged TQ interval.
| [Taboo]
1.The allergic to this product;
2.Patients suffer serious dysfunction of heart, liver, kidney (relative contraindication);
3.Pregnant women, lactating women, children, the old and co-infected HIV sufferers (relative contraindication). |
|
|