| Identification | More | [Name]
1,4-DIBROMONAPHTHALENE | [CAS]
83-53-4 | [Synonyms]
1,4-DIBROMONAPHTHALENE
1,4-DIBROMONAPTHALENE
1,4-dibromo-naphthalen | [EINECS(EC#)]
201-484-8 | [Molecular Formula]
C10H6Br2 | [MDL Number]
MFCD00041823 | [Molecular Weight]
285.96 | [MOL File]
83-53-4.mol |
| Chemical Properties | Back Directory | [Appearance]
light orange-beige fine crystalline powder | [Melting point ]
80-82 °C
| [Boiling point ]
288.1°C (rough estimate) | [density ]
1.8199 (estimate) | [refractive index ]
1.6000 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform, DMSO (Slightly), Methanol (Slightly) | [form ]
Crystalline Powder or Crystals | [color ]
White to light beige | [Water Solubility ]
347.9ug/L(25 ºC) | [InChIKey]
IBGUDZMIAZLJNY-UHFFFAOYSA-N | [CAS DataBase Reference]
83-53-4(CAS DataBase Reference) | [EPA Substance Registry System]
Naphthalene, 1,4-dibromo- (83-53-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [TSCA ]
Yes | [HazardClass ]
IRRITANT | [HS Code ]
29039990 |
| Hazard Information | Back Directory | [Chemical Properties]
light orange-beige fine crystalline powder | [Uses]
1,4-Dibromonaphthalene is used in the annelation of PAHs. Also used in the synthesis of novel, orally active dual NK1/NK2 antagonists. | [Application]
1,4-Dibromonaphthalene is mainly used for pharmaceutical intermediates. synthesis of tribromo methoxynaphthalenes from 1,4-dibromonaphthalene. starting material 1,4-dibromonaphthalene was prepared starting from naphthalene or 1-bromonaphthalene.
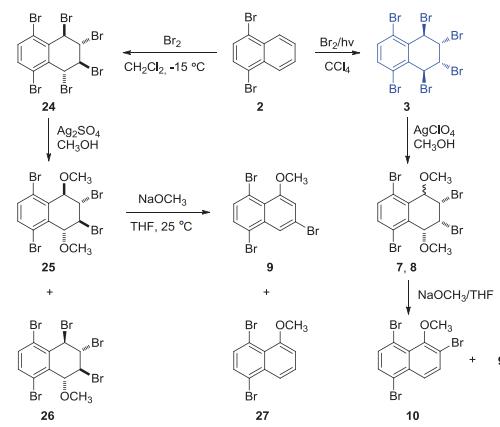
5,8-Dibromo-1,4-naphthoquinone and 5,8-diiodo-1,4-naphthoquinone were prepared from 1,4-dibromonaphthalene and 1,4-diiodonaphthalene. | [Preparation]
An environment-friendly method was developed to synthesize 1,4-dibromonaphthalene (1,4-DBN) using 1,3-dialkylimidazolium and pyridinium ionic liquids as catalysts, over which the yields of 1,4-DBN were obtained as high as 100%.
An environment-friendly method for synthesis of 1,4-dibromo-naphthalene in aqueous solution of ionic liquids |
|
|