| Identification | More | [Name]
(4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE | [CAS]
77943-39-6 | [Synonyms]
(4R,5S)-(-)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE
(4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE
(4R,5S)-4-METHYL-5-PHENYL-2-OXAZOLIDINONE
4-Methyl-5-phenyl-1,3-oxazolidin-2-one
(4R,5S)-4-Methyl-5-phenyl-2-oxazolidone
(4R,5S)-(+)-4-METHYL-5-PHENYL-2-OXAZOLID-INONE, 99% (99% EE/GLC)
(4R,5S)-4-METHYL-5-PHENYL-2-OXAZOLIDI DI NONE
2-Oxazolidinone, 4-methyl-5-phenyl-, (4R,5S)-
4-methyl-5-phenyl-2-oxazolidinone
(4R)-4α-Methyl-5α-phenyloxazolidin-2-one
4α-Methyl-5α-phenyloxazolidine-2-one
5β-Phenyl-4β-methyloxazolidine-2-one
(4S)-4β-Methyl-5α-phenyloxazolidine-2-one
(4S,5S)-4-Methyl-5-phenyloxazolidin-2-one | [EINECS(EC#)]
660-040-4 | [Molecular Formula]
C10H11NO2 | [MDL Number]
MFCD00010845 | [Molecular Weight]
177.2 | [MOL File]
77943-39-6.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powde | [Melting point ]
121-123 °C(lit.)
| [Boiling point ]
309.12°C (rough estimate) | [density ]
1.1607 (rough estimate) | [refractive index ]
1.5168 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly) | [form ]
Liquid | [pka]
12.35±0.60(Predicted) | [color ]
Clear colorless to yellow-brown, may darken in storage | [optical activity]
[α]18/D +168°, c = 2 in chloroform | [BRN ]
1211705 | [InChI]
InChI=1S/C10H11NO2/c1-7-9(13-10(12)11-7)8-5-3-2-4-6-8/h2-7,9H,1H3,(H,11,12)/t7-,9-/m1/s1 | [InChIKey]
PPIBJOQGAJBQDF-VXNVDRBHSA-N | [SMILES]
O1[C@@H](C2=CC=CC=C2)[C@@H](C)NC1=O | [CAS DataBase Reference]
77943-39-6(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
N | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [HS Code ]
29349990 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal powde | [Uses]
(4R,5S)-4-Methyl-5-phenyloxazolidinone is used as effective chiral auxiliary for conjugate addition asymmetric synthesis of (-)-aplysillamide B. | [Definition]
ChEBI: (4R,5S)-(+)-4-Methyl-5-phenyl-2-oxazolidinone is a member of benzenes. | [Preparation]
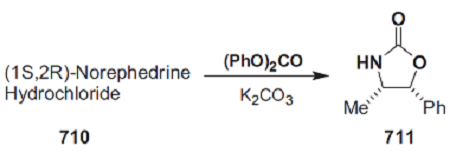
A mechanically stirred mixture of (1S,2R)-norephedrine 710 (151 g, 1.00 mol) ([α]589 =+ 33.4 (c= 7, water)), as the hydrochloride salt, diphenyl carbonate (236 g, 1.10 mol), and anhydrous potassium carbonate (152 g, 1.10 mol) was heated at 110 °C for 4–6 h. The resultant mixture was then cooled to <60 °C. Excess diphenyl carbonate was hydrolyzed by adding methanol (600 mL) and heating the mixture under reflux for 0.5 h. Sufficient water (400–600 mL) was then added to dissolve the potassium carbonate. Methanol was removed in vacuo. The product and phenol were extracted into dichloromethane (3 × 1 L). The combined extracts were washed with 2 m aqueous sodium hydroxide (3 × 1 L) to remove the phenol, 1 m aqueous hydrochloric acid (1 × 1 L), and brine, dried over anhydrous magnesium sulfate, and concentrated in vacuo to give 195 g (110% mass balance) of a light-yellow solid. Recrystallization from toluene (600 mL) afforded 145–165 g (82–93%) of oxazolidinone 711 as a white crystalline solid.
| [Preparation]
To a solution of (1S,2R)-norephedrine (40 g, 0.26 mol) in toluene (400 mL) was added diethyl carbonate (37 mL, 0.32 mol). The mixture was heated to reflux (under Ar) while 40 mL of solvent was removed through the use of a Dean–Stark apparatus. The mixture was allowed to cool for 20 min, and then sodium methoxide (1 g) was added. Upon reheating, an EtOH/toluene azeotropic mixture was removed at 75–77 °C. After 3 h, the reaction was complete and the temperature of the mixture had increased to 125 °C. The mixture was left to stand at room temperature for 16 h, whereupon (4R,5S)-4-methyl-5-phenyloxazolidin-2-one (40.6 g) crystallized and could be collected. The solvent was removed from the filtrate in vacuo and the residue was redissolved in EtOAc (250 mL). This solution was washed with brine (50 mL) and a precipitate was removed by filtration. The solvent was then removed in vacuo and toluene (50 mL) was added to the residue. Removal of the toluene by distillation yielded oily crystals of the oxazolidinone, which were washed with Et2O to afford 4.5 g (total 45 g, 97%). | [Synthesis Reference(s)]
Tetrahedron Letters, 40, p. 6059, 1999 DOI: 10.1016/S0040-4039(99)01256-3 |
|
|