| Identification | More | [Name]
CHROMIUM(III) FLUORIDE | [CAS]
7788-97-8 | [Synonyms]
CHROMIC FLUORIDE
CHROMIUM FLUORIDE
CHROMIUM(III) FLUORIDE
Chromium(III) trifluoride
CHROMIUM TRIFLUORIDE
Chrome fluorure
Chromic trifluoride
chromicfluoride,solid
chromicfluoride,solution
Chromium fluoride (CrF3)
CrF3
Fluorchrome
Chromiumfluorideanhydrousgreenpowder
CHROMIUM(III) FLUORIDE, ANHYDROUS, POWDE R, 99.99%
Chromium(III)fluoride,ultradry,99.99%(metalsbasis)
Chromiumtrifluoride,anhydrous
Chromium(III)fluoride,anhydrous,98%
chromium(iii) fluoride, ultra dry
Chromium(III) fluoride anhydrous 99%
Chromium(III)fluorideanhydrous99% | [EINECS(EC#)]
232-137-9 | [Molecular Formula]
CrF3 | [MDL Number]
MFCD00016045 | [Molecular Weight]
108.99 | [MOL File]
7788-97-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Green crystalline powder or crystals | [Melting point ]
>1000°C | [density ]
3.8 g/mL at 25 °C(lit.)
| [vapor pressure ]
0Pa at 20℃ | [solubility ]
insoluble in H2O, ethanol | [form ]
powder
| [color ]
Green | [Water Solubility ]
insoluble H2O; soluble HCl, violet color [MER06] | [Sensitive ]
Hygroscopic | [Merck ]
14,2223 | [Exposure limits]
ACGIH: TWA 2.5 mg/m3
NIOSH: IDLH 250 mg/m3; IDLH 25 mg/m3; TWA 0.5 mg/m3 | [LogP]
-2 at 20℃ | [Uses]
Printing and dyeing woolens, moth-proofing,
halogenation catalyst. | [CAS DataBase Reference]
7788-97-8(CAS DataBase Reference) | [EPA Substance Registry System]
Chromium fluoride (CrF3) (7788-97-8) |
| Hazard Information | Back Directory | [Chemical Properties]
Green crystalline powder or crystals | [General Description]
Chromic fluoride solution is a green crystalline solid dissolved in water. CHROMIC FLUORIDE, SOLUTION(7788-97-8) is corrosive to metals and tissue. | [Reactivity Profile]
Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. | [Air & Water Reactions]
Water soluble. | [Hazard]
Irritant to skin and eyes, especially in solution. | [Health Hazard]
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | [Fire Hazard]
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. | [Physical properties]
Dark green needles (anhydrous salt) or green hexagonal crystals (trihydrate); density 3.8 g/cm3 (anhydrous fluoride), 2.2 g/cm3 (trihydrate); anhydrous salt melts at 1,100°C and sublimes above this temperature; practically insoluble in water and ethanol (anhydrous salt); trihydrate sparingly soluble in water; soluble in HCl forming a violet solution. | [Flammability and Explosibility]
Notclassified |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R31:Contact with acids liberates toxic gas.
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S27:Take off immediately all contaminated clothing .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 1756 8/PG 2
| [WGK Germany ]
3
| [RTECS ]
GB6125000
| [Hazard Note ]
Corrosive | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
28261990 | [Safety Profile]
A poison by ingestion.
A corrosive. When heated to decomposition
it emits toxic vapors of Cr and F-. |
| Questions And Answer | Back Directory | [Chemical Properties]
Chromium(III) fluoride [7788-97-8], CrF3, Mr 108.99. The anhydrous compound forms highly refractive rhombohedral crystals. It melts above 1000°C and is distinctly volatile between 1100 and 1200 °C. Chromium(III) fluoride is insoluble in water if no divalent chromium is present. Double compounds are formed with other metal fluorides, e.g., green CrF3 · 2KF·H2O.

Hydrates are known which contain three [16671-27-5] to nine [68374-27-6] molecules of water. The violet hexaaquochromium(III) fluoride, [Cr(H2O)6]F3, and its trihydrate, [Cr(H2O)6]F3 · 3H2O, can be obtained from hexaaquochromium(III) salt solutions and alkali fluorides. Products containing lesswater are green. The composition of the industrial product corresponds approximately to CrF3 · 3.5H2O and the product contains about 30 % chromium.
For the production of chromium(III) fluoride hydrate, chromium(III) oxide hydrate is dissolved in hot aqueous hydrofluoric acid and the green salt crystallizes. Chromium(III) fluoride is used in the textile industry for mordanting wool, for chromating dyestuffs, and in vigoureux printing. Chromium(III) fluorides have also found application in rust-prevention paints as corrosion inhibitors.
| [Uses]
Some important uses are in printing and dyeing woolens; mothproofing of woolen materials; metal polishing; coloring marbles; and as a catalyst in halogenation reactions.
| [Preparation]
Chromium(III) fluoride may be prepared by heating chromium trichloride under a stream of hydrogen fluoride:
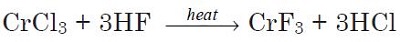
The compound may be prepared by the reaction of chromium hydroxide with hydrofluoric acid:
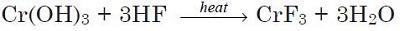
|
|
|