| Identification | Back Directory | [Name]
2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]-1-(p-toluenesulfonyl)-ethanol | [CAS]
77544-60-6 | [Synonyms]
PEG5-Tos
Tos-PEG4
Tos-PEG4-OH
Hydroxy-PEG5-Tos
Tetraethylene glycol monotosylate
Monotosylated tetraethylene glycol
Tetraethylene glycol p-toluenesulfonate
Tetraethylene glycol p-toluenesulfonate 97%
2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethyl tosylate
2-(2-(2-(2-hydroxyethoxy)ethoxy)ethoxy)-1-tosylethanol
2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethyl p-Toluenesul
2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]-1-(p-toluenesulfonyl)
2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethyl toluenesulfonate
2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethyl toluene-4-sulfonate
2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]-1-(p-toluenesulfonyl)-ethanol
2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethyl 4-methylbenzenesulfonate
Ethanol,2-[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]-,4-methylbenzenesulfonate
p-Toluenesulfonic Acid 2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethyl Ester
Ethanol,2-[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]-, 1-(4-Methylbenzenesulfonate) | [Molecular Formula]
C15H24O7S | [MDL Number]
MFCD18433414 | [MOL File]
77544-60-6.mol | [Molecular Weight]
348.41 |
| Chemical Properties | Back Directory | [Boiling point ]
492.5±40.0 °C(Predicted) | [density ]
1.202 g/mL at 25 °C | [refractive index ]
n20/D1.507 | [Fp ]
>110℃ | [storage temp. ]
2-8°C | [solubility ]
Soluble in Water, DMSO, DCM, DMF | [form ]
Oil | [pka]
14.36±0.10(Predicted) | [color ]
Colourless |
| Hazard Information | Back Directory | [Description]
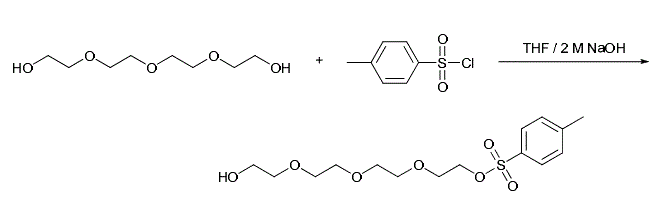
PEG5-Tos is a PEG linker containing a hydroxyl group with a tosyl group. The hydrophilic PEG spacer increases solubility in aqueous media. The hydroxyl group enables further derivatization or replacement with other reactive functional groups. The tosyl group is a very good leaving group for nucleophilic substitution reactions. | [Uses]
Tetraethylene glycol monotosylate is a cleavable and acylhydrazone-based ADC linker used in the synthesis of antibody-drug conjugates (ADCs). Tetraethylene glycol monotosylate also can be used as a PROTAC linker that can be used in the synthesis of PROTACs. | [Preparation]
Synthesis of tetraethylene glycol tosylate. To a solution of tetraethylene glycol (2.22 g, 11.4 mmol, 3.8 equiv) in THF at 0 °C was added 2.5 M NaOH (2 mL, 5.0 mmol, 1.7 equiv) and stirred for 30 min. Tosyl chloride (565.3 mg, 3.0 mmol, 1 equiv) was added in small portions and the mixture stirred for 5 h. The solvent was removed under reduced pressure, and the residue was taken up in CH2Cl2 (15 mL) and washed with water (3 × 10 mL). The aqueous wash was further extracted with CH2Cl2 (3 × 10 mL) and the combined organic fractions were washed with brine and dried over anhydrous Na2SO4. The dried solution was filtered and the filtrate was concentrated under reduced pressure to give 900.8 mg of a pale yellow oil (87%). The product was sufficiently pure to be used without further purification.
 |
|
|