| Identification | More | [Name]
Boron trifluoride | [CAS]
7637-07-2 | [Synonyms]
BORON FLUORIDE
BORON TRIFLUORIDE
BORON TRIFLUORIDE GAS
anca1040
BF3
Borane,trifluoro-
Boron fluoride (BF3)
boronfluoride(bf3)
Fluorure de bore
fluoruredebore
fluoruredebore(french)
Leecure b series
trifluoro-boran
Trifluoroborane
trifluoro-Borane
Trifluoroboron
BORON TRIFLUORIDE, 99.5+%
BORON TRIFLUORIDE, 99.99+%, ELECTRONIC G RADE
BORON TRIFLUORIDE (~10% (~1.3 M) IN 1-BUTANOL)
BORON TRIFLUORIDE CYL. WITH 2 L (NET ~1. 2 KG) | [EINECS(EC#)]
206-766-4 | [Molecular Formula]
BF3 | [MDL Number]
MFCD00011316 | [Molecular Weight]
67.81 | [MOL File]
7637-07-2.mol |
| Chemical Properties | Back Directory | [Description]
Dry boron trifluoride is used with mild steel, copper, copper–zinc
and copper–silicon alloys, and nickel. Moist gas is corrosive to most metallic materials
and some plastics. Therefore, Kel-F? and Teflon? are the preferred gasketing materials.
Mercury-containing manometers should not be used since boron trifluoride is soluble
in mercury. It decomposes in hot water, yielding hydrogen fluoride. Boron trifluoride is
widely used as a catalyst for organic synthesis reactions. | [Appearance]
Boron trifluoride is a nonflammable, colorless
gas. Pungent, suffocating odor. Forms dense white fumes in
moist air. Shipped as a nonliquefied compressed gas. | [Melting point ]
−127 °C(lit.) | [Boiling point ]
−100 °C(lit.) | [density ]
0.87 g/mL at 20 °C | [vapor density ]
2.38 (21 °C, vs air)
| [vapor pressure ]
>1 mmHg at 20 °C | [refractive index ]
n20/D 1.38
| [Fp ]
4°C | [storage temp. ]
2-8°C
| [solubility ]
soluble in H2O | [form ]
Liquid | [color ]
Colorless | [Odor]
Pungent odor detectable at 1.5 ppm | [Water Solubility ]
MAY DECOMPOSE | [Sensitive ]
Moisture Sensitive | [Merck ]
14,1349 | [Exposure limits]
ACGIH: TWA 0.1 ppm(2.5 mg/m3); Ceiling 0.7 ppm
OSHA: Ceiling 1 ppm(3 mg/m3)
NIOSH: IDLH 25 ppm(250 mg/m3); Ceiling 1 ppm(3 mg/m3) | [LogP]
1.070 (est) | [CAS DataBase Reference]
7637-07-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Borane, trifluoro-(7637-07-2) | [EPA Substance Registry System]
Boron trifluoride (7637-07-2) |
| Safety Data | Back Directory | [Hazard Codes ]
T+,C,T,F | [Risk Statements ]
R14:Reacts violently with water.
R26:Very Toxic by inhalation.
R35:Causes severe burns.
R39/23/24/25:Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed .
R24/25:Toxic in contact with skin and if swallowed .
R11:Highly Flammable.
R67:Vapors may cause drowsiness and dizziness.
R41:Risk of serious damage to eyes.
R10:Flammable. | [Safety Statements ]
S9:Keep container in a well-ventilated place .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S16:Keep away from sources of ignition-No smoking . | [OEL]
Ceiling: 1 ppm (3 mg/m3) | [RIDADR ]
UN 3286 3/PG 2
| [WGK Germany ]
3
| [RTECS ]
ED2275000
| [F ]
21 | [Hazard Note ]
Corrosive/Toxic | [TSCA ]
Yes | [DOT Classification]
2.3, Hazard Zone B (Gas poisonous by inhalation) | [HazardClass ]
2.3 | [Precautions]
Exposures to boron trifl uoride in occupational work areas cause irritating effects,
painful burns, lesions, and loss of vision. Workers with potential exposure to boron
trifl uoride should not wear contact lenses. Prompt medical attention is mandatory
in all cases of overexposure to boron trifl uoride and the rescue personnel should be
equipped with proper protectives. Occupational workers should handle/use boron trifl uoride only in well-ventilated areas. The valve protection caps must remain in place.
Workers should not drag, slide, or roll the cylinders, and use a suitable hand truck for
cylinder movement. Compressed gas cylinders shall not be refi lled without the express
written permission of the owner. Boron trifl uoride is listed as an extremely hazardous
substance (EHS).
The cylinder should not be heated by any means to increase the discharge rate of the
product from the cylinder. The cylinder of boron trifl uoride should be kept stored in a cool,
dry, well-ventilated area of non-combustible construction away from heavily traffi cked
areas and emergency exits | [Hazardous Substances Data]
7637-07-2(Hazardous Substances Data) | [Toxicity]
LC50 inhal (rat) 387 ppm (1070 mg/m3; 1 h)
PEL (OSHA) 1 ppm (3 mg/m3; ceiling)
TLV (ACGIH) 1 ppm (3 mg/m3; ceiling) | [IDLA]
25 ppm |
| Hazard Information | Back Directory | [General Description]
BORON TRIFLUORIDE(7637-07-2) is a colorless gas with a pungent odor. BORON TRIFLUORIDE(7637-07-2) is toxic by inhalation. BORON TRIFLUORIDE(7637-07-2) is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material. Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing. | [Reactivity Profile]
BORON TRIFLUORIDE is a colorless, strongly irritating, toxic gas. Upon contact with water, steam or when heated to decomposition, BORON TRIFLUORIDE will produce toxic fluoride fumes. Incompatible with alkyl nitrates, calcium oxide. Reaction with alkali metals or alkaline earth metals (except magnesium) will cause incandescence [Bretherick, 5th ed., 1995, p. 65]. | [Air & Water Reactions]
Fumes in air. Soluble in water and slowly hydrolyzed by cold water to give hydrofluoric acid. Reacts more rapidly with hot water. | [Hazard]
Toxic by inhalation, corrosive to skin and
tissue. Lower respiratory tract irritant, and pneu-
monitis.
| [Health Hazard]
Exposures to boron trifl uoride cause irritating effects, painful burns, lesions, loss of vision,
stinging of the skin, irritation of the upper respiratory tract, and cough. Higher concentrations may cause infl ammation and congestion of the lungs. Occupational exposures
to high concentrations of boron trifl uoride result in burns to the mucous membranes.
Even at low concentrations (as low as 50 ppm), boron trifl uoride causes cardiac collapse,
pulmonary edema, and chemical pneumonitis. | [Health Hazard]
Highly toxic; may cause death or permanent injury after very short exposure to small quantities. Substance is irritating to the eyes, the skin, and the respiratory tract. | [Potential Exposure]
Boron trifluoride is a highly reactive
chemical used primarily as a catalyst in chemical synthesis.
It is stored and transported as a gas, but can be reacted
with a variety of materials to form both liquid and solid
compounds. The magnesium industry utilizes the fireretardant
and antioxidant properties of boron trifluoride
in casing and heat treating. Nuclear applications of boron
trifluoride include neutron detector instruments; boron-10
enrichment and the production of neutroabsorbing salts for
molten-salt breeder reactors. | [Fire Hazard]
When heated to decomposition or upon contact with water or steam, BORON TRIFLUORIDE will produce toxic and corrosive fumes of fluorine containing compounds. Decomposes upon heating or on contact with moist air, forming toxic and corrosive fumes of boric acid and hydrofluoric acid. Reacts with alkalis and fumes in moist air, producing particulates which reduce visibility. Reacts with alkali metals, alkaline earth metals (except magnesium), alkyl nitrates, and calcium oxide. BORON TRIFLUORIDE hydrolyzes in moist air to form boric acid, hydrofluoric acid, and fluoboric acid. | [First aid]
If contact with liquid, treat for frostbite. If this
chemical gets into the eyes, remove any contact lenses at
once and irrigate immediately for at least 30 minutes, occasionally
lifting upper and lower lids. Seek medical attention
immediately. If this chemical contacts the skin, remove
contaminated clothing and wash immediately with soap and
water. Seek medical attention immediately. If this chemical
has been inhaled, remove from exposure, begin rescue
breathing (using universal precautions, including resuscitation
mask) if breathing has stopped and CPR if heart action
has stopped. Transfer promptly to a medical facility. When
this chemical has been swallowed, get medical attention.
Give large quantities of water and induce vomiting. Do not
make an unconscious person vomit. Medical observation is
recommended for 24 to 48 hours after breathing overexposure,
as pulmonary edema may be delayed. As first aid for
pulmonary edema, a doctor or authorized paramedic may
consider administering a drug or other inhalation therapy. | [Shipping]
UN1008 Boron trifluoride, Hazard class: 2.3;
Labels: 2.3—Poisonous gas, 8—Corrosive material,
Inhalation Hazard Zone B. Cylinders must be transported
in a secure upright position, in a well-ventilated truck.
Protect cylinder and labels from physical damage. The
owner of the compressed gas cylinder is the only entity
allowed by federal law (49CFR) to transport and refill
them. It is a violation of transportation regulations
to refill compressed gas cylinders without the express
written permission of the owner. | [Incompatibilities]
Boron trifluoride reacts with polymerized
unsaturated compounds. Decomposes on contact with
water, moist air, and other forms of moisture, forming toxic
and corrosive hydrogen fluoride, fluoroboric acid, and boric
acid. Reacts violently with alkali and alkaline earth metals
(except magnesium); metals, such as sodium, potassium,
and calcium oxide, and with alkyl nitrates. Attacks many
metals in presence of water. | [Waste Disposal]
Return refillable compressed
gas cylinders to supplier. The owner of the compressed gas
cylinder is the only entity allowed by federal law (49CFR)
to transport and refill them. Chemical reaction with water
to form boric acid, and fluoroboric acid. The fluoroboric
acid is reacted with limestone, forming boric acid and calcium
fluoride. The boric acid may be discharged into a sanitary
sewer system while the calcium fluoride may be
recovered or landfilled. Protect cylinder and labels from
physical damage. | [Physical properties]
Colorless gas; pungent suffocating odor; density 2.975 g/L; fumes in moist air; liquefies at -101°C; solidifies at -126.8°; vapor pressure at -128°C is 57.8 torr; critical temperature -12.2°C; critical pressure 49.15 atm; critical volume 115 cm3/mol; soluble in water with partial hydrolysis; solubility in water at 0°C 332 g/100g; also soluble in benzene, toluene, hexane, chloroform and methylene chloride; soluble in anhydrous concentrated sulfuric acid. | [Uses]
Boron trifluoride is used as a catalyst for polymerizations,
alkylations, and condensation
reactions; as a gas flux for internal soldering or
brazing; and as a source of B10 isotope. | [Uses]
In catalysis with and without promoting
agents; fumigant; flux for soldering
magnesium | [Uses]
To protect molten magnesium and its alloys from oxidation; as a flux for soldering magnesium; as a fumigant; in ionization chambers for the detection of weak neutrons. By far the largest application of boron trifluoride is in catalysis with and without promoting agents. | [Definition]
ChEBI: Boron trifluoride is a boron fluoride. | [Preparation]
Boron trifluoride is prepared by treating borax with hydrofluoric acid; or boric acid with ammonium bifluoride. The complex intermediate product is then treated with cold fuming sulfuric acid. | [Flammability and Explosibility]
Boron trifluoride gas is noncombustible. Water should not be used to extinguish any
fire in which boron trifluoride is present. Dry chemical powder should be used for
fires involving organic complexes of boron trifluoride. | [Materials Uses]
Dry boron trifluoride does not react with the
common metals of construction, but If moisture
is present the acidic hydrates formed (BF3·H2O
and BF3·2H2O) can corrode many common metals
rapidly. Consequently, lines, pressure regulators,
and valves in boron trifluoride service
must be well protected from the entrance of
moist air between periods of use. Cast iron must
not be used because active fluorides attack its
structure. If steel piping is used for boron
trifluoride, forged-steel fittings must be used
instead of cast-iron fittings. Stainless steel, Monel,
nickel, and Hastelloy C are good materials
of construction.
Among materials suitable for gaskets are
Teflon, Kel F, and other appropriate fluorocarbon
or chlorofluorocarbon plastics. Most plastics
become embrittled in boron trifluoride
service. The use of polyvinyl chloride should be
avoided. | [Physiological effects]
Boron trifluoride irritates the nose, mucous
membranes, and other parts of the respiratory
system. Concentrations as low as I ppm in air
can be detected by the sense of smell and are
readily visible.
ACGIH recommends a Threshold Limit
Value-Ceiling (TLV-C) of 1 ppm (2.8 mg/m3)
for boron trifluoride. The TLV-C is the concentration
that should not be exceeded during
any part of the working exposure. | [storage]
All work with boron trifluoride should be conducted in a
fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves
should be worn to prevent eye and skin contact. Cylinders of boron trifluoride should be
stored in locations appropriate for compressed gas storage and separated from alkali metals,
alkaline earth metals, and other incompatible substances. Solutions of boron trifluoride should
be stored in tightly sealed containers under an inert atmosphere in secondary containers. | [Purification Methods]
The usual impurities-bromine, BF5, HF and non-volatile fluorides-are readily separated by distillation. Brown and Johannesen [J Am Chem Soc 72 2934 1950] passed BF3 into benzonitrile at 0o until the latter was saturated. Evacuation to 10-5mm then removed all traces of SiF4 and other gaseous impurities. [A small amount of the BF3-benzonitrile addition compound sublimes and is collected in a U-tube cooled to -80o]. The pressure is raised to 20mm by admitting dry air, and the flask containing the BF3 addition compound is warmed with hot water. The BF3 that evolves is passed through a -80o trap (to condense any benzonitrile) into a tube cooled in liquid air. The addition compound with anisole can also be used. BF3 can be dried by passing it through H2SO4 saturated with boric oxide. It fumes in moist air. [It is commercially available as a 1.3M solution in MeOH or PrOH.] [Booth & Wilson Inorg Synth I 21 1939, Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 219-222 1963.] TOXIC. | [Toxics Screening Level]
The Initial Threshold Screening Level (ITSL) for boron trifluoride is 0.7 μg/m3 based on a 24 hr. averaging time. | [GRADES AVAILABLE]
Boron trifluoride is available for commercial
and industrial use in technical grades having
much the same component proportions from one
producer to another.
Boron trifluoride is also available in
high-purity grades for use in the electronics
industry. Gas purity guidelines have been developed
and published by the Semiconductor
Equipment and Materials International and can
be found in the Book ofSEMI Standards, Gases
Volume. |
| Questions And Answer | Back Directory | [Description]
Boron trifluoride is the inorganic compound with the formula BF3. It is a highly toxic, colorless and nonflammable gas with a penetrating and pungent odor. It dissolves quickly in water and any organic compounds containing nitrogen or oxygen. It can be slowly hydrolyzed by cold water to give off hydrofluoric acid, and can also hydrolyzes to form white dense fumes in moist air. Its vapors are heavier than air. Inhaling the gas will irritate the respiratory system and burns can result if the gas touches the skin in high concentrations.
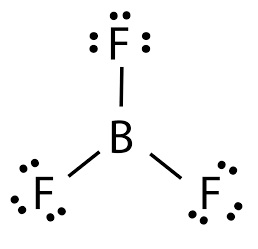
boron trifluoride lewis structure
Boron trifluoride is most importantly used as a reagent, typically as a Lewis acid, to catalyze such diverse operations as isomerization, alkylation, polymerization, esterfication, condensation, cyclization, hydration dehydration, sulfonation, desulfurization nitration, halogenation oxidation and acylation. Besides, it can also be used as a versatile building block for other boron compounds. | [Chemical Properties]
Boron trifluoride, BF3, is a colorless gas with a vapor density of 2.34, which is heavier than air. It is water-soluble and does not support combustion. It is also water-reactive, toxic by inhalation, and corrosive to skin and tissue. The TLV is 1 ppm, and the IDLH is 100 ppm in air. The boiling point is ?148°F (64°C). The four-digit UN identification number is 1008. The NFPA 704 designation is health 4, flammability 0, and reactivity 1. The primary uses are as a catalyst in organic synthesis, in instruments for measuring neutron intensity, in soldering fluxes, and in gas brazing. | [References]
1. https://en.wikipedia.org/wiki/Boron_trifluoride
2. https://cameochemicals.noaa.gov/chemical/255
3. http://www.c-f-c.com/specgas_products/boron-trifluoride.htm
4.https://www.praxairdirect.com/Specialty-Gas-Information-Center/Pure-Gas-Specifications/Boron-Trifluoride.html
|
|