| Identification | More | [Name]
Trichloroacetic acid | [CAS]
76-03-9 | [Synonyms]
RARECHEM AL BO 0072
TCA
TRICHLOROACETIC ACID
Aceticacid,trichloro-
Aceto-Caustin
Acide trichloracetique
acidetrichloracetique
acidetrichloracetique(french)
acidetrichloracetique(solide)
acidetrichloracetique(solutions)
Acido tricloroacetico
acidotricloroacetico
Amchem Grass Killer
amchemgrasskiller
CCl3COOH
Farmon TCA
Konesta
Kyselina trichloroctova
kyselinatrichloroctova
NA TA | [EINECS(EC#)]
200-927-2 | [Molecular Formula]
C2HCl3O2 | [MDL Number]
MFCD00004177 | [Molecular Weight]
163.39 | [MOL File]
76-03-9.mol |
| Chemical Properties | Back Directory | [Appearance]
Trichloroacetic acid is a colorless crystalline solid which is used in liquid solutions. | [Melting point ]
54-58 °C (lit.) | [Boiling point ]
196 °C (lit.) | [bulk density]
900kg/m3 | [density ]
1.62 g/mL at 25 °C(lit.)
| [vapor density ]
<1 (vs air)
| [vapor pressure ]
1 mm Hg ( 51 °C)
| [refractive index ]
n20/D 1.62(lit.)
| [Fp ]
196°C | [storage temp. ]
2-8°C
| [solubility ]
H2O: 0.5 M at 20 °C, clear, colorless
| [form ]
Solid | [pka]
0.7(at 25℃) | [color ]
White | [Odor]
sharp, pungent odor | [PH]
<1.0 (25℃, 0.5M in H2O) | [Stability:]
Stable, but moisture sensitive. Incompatible with water, strong bases. Note that the Merck Index states that this material is hydrolytically unstable in aqueous solution below 30% by weight. Decomposition products include carbon monoxide and carbon dioxide. The generation of these gases in a sealed container may lead to a pressure rise sufficien | [Water Solubility ]
120 g/100 mL (20 ºC) | [Sensitive ]
Hygroscopic | [λmax]
210nm(EtOH)(lit.) | [Merck ]
14,9627 | [BRN ]
970119 | [Dielectric constant]
4.9(20℃) | [Exposure limits]
ACGIH: TWA 0.5 ppm
NIOSH: TWA 1 ppm(7 mg/m3) | [LogP]
1.330 | [CAS DataBase Reference]
76-03-9(CAS DataBase Reference) | [IARC]
2B (Vol. 63, 84, 106) 2014 | [NIST Chemistry Reference]
Acetic acid, trichloro-(76-03-9) | [EPA Substance Registry System]
76-03-9(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,N,C,F | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R40:Limited evidence of a carcinogenic effect.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R35:Causes severe burns.
R38:Irritating to the skin.
R11:Highly Flammable.
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S60:This material and/or its container must be disposed of as hazardous waste .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S24/25:Avoid contact with skin and eyes . | [OEB]
B | [OEL]
TWA: 1 ppm (7 mg/m3) | [RIDADR ]
UN 1839 8/PG 2
| [WGK Germany ]
2
| [RTECS ]
AJ7875000
| [F ]
10-21 | [Autoignition Temperature]
711 °C | [Hazard Note ]
Corrosive/Hygroscopic | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29154000 | [Safety Profile]
Poison by ingestion and
subcutaneous routes. Moderately toxic by
intraperitoneal route. Questionable
carcinogen with experimental carcinogenic
data. Experimental reproductive effects.
Mutation data reported. A corrosive irritant
to skin, eyes, and mucous membranes.
When heated to decomposition it emits
toxic fumes of Cland Na2O. Used as an
herbicide. | [Hazardous Substances Data]
76-03-9(Hazardous Substances Data) | [Toxicity]
LD50 orally in rats: 5000 mg/kg (Bailey, White) |
| Hazard Information | Back Directory | [General Description]
TRICHLOROACETIC ACID(76-03-9), solid is a colorless crystalline solid. TRICHLOROACETIC ACID(76-03-9) absorbs moisture from air and forms a syrup. TRICHLOROACETIC ACID(76-03-9) is soluble in water with release of heat. TRICHLOROACETIC ACID(76-03-9) is corrosive to metals and tissue. | [Reactivity Profile]
TRICHLOROACETIC ACID is a strong acid; when heated, in the presence of water, decomposes forming phosgene and HCl. [Handling Chemicals Safely 1980 p. 915]. The acid was added to copper wool and rinsed down with dimethyl sulfoxide. This caused what was thought to be an extremely exothermic dehydrohalogenation reaction that melted the neck of the flask, [Chem. Eng. News, 1981, 59(28), 4]. | [Air & Water Reactions]
TRICHLOROACETIC ACID is soluble in water with release of heat. | [Health Hazard]
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | [Potential Exposure]
This haloacetic acid can be a byproduct of drinking water disinfection and may increase the risk of cancer. Trichloroacetic acid is used as medication; in organic syntheses; as a reagent for albumin detection; as an intermediate in pesticide manufacture and in the production of sodium trichloroacetate which is itself a herbicide. | [Fire Hazard]
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water, or milk. Do not induce vomiting. Medical observation isrecommended for 24-48 hours after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a drug or other inhalation therapy. | [Shipping]
UN1839 (solid) & UN2564 (solution) Trichloroacetic acid, solid and Trichloroacetic acid, solution, Hazard class: 8; Labels: 8-Corrosive material. | [Incompatibilities]
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, silver salts, strong acids, strong bases, moisture, iron, zinc, aluminum. Corrosive to iron, steel and other metals. | [Chemical Properties]
Trichloroacetic acid is a colorless crystalline solid which is used in liquid solutions. | [Chemical Properties]
Trichloroacetic acid, is a colorless crystalline solid. Trichloroacetic acid absorbs moisture from air and forms a syrup. Trichloroacetic acid is soluble in water with release of heat. Trichloroacetic acid is corrosive to metals and tissue.
| [Uses]
As a reagent for albumin detection; in
making herbicides. It is found as a by-product
after chlorination of water containing humic
materials. | [Uses]
Traditionally used to precipitate protein. Has been used to determine protein concentration by quantitative precipitation. Used as decalcifier and fixative, as a laboratory reagent, a herbicide, in medicine, and in microscopy.
| [Uses]
Trichloroacetate (TCA) is used primarily for the selective control of annual and perennial grass weeds in cropland and noncropland. TCA is acidic in nature and are not strongly sorbed by soils. It is reported to be rapidly degraded in both soil and water by microbial processes. However, the breakdown of TCA occurs very slowly when incubated at 14–15 °C in acidic soils. iming not only accelerates this degradation but also increases the numbers of TCA-degrading bacteria. | [Application]
Trichloroacetic acid can be used as pharmaceutical raw materials, herbicides (potassium trichloroacetate and sodium trichloroacetate, etc.), textile dyeing auxiliaries, metal surface treatment agent and acid chloride, anhydride, amide, polyester, organometallic salt, water salicylaldehyde, chlorocarboxylic acid and the raw materials of other organic synthesis.
In addition, in medicine, it can also be used as etherifying agents and keratolytics, bile pigment reagents and protein precipitation reagents. In the field of biochemistry, it can be used for separation analysis of biological phosphate compounds and reagents for determination of fluoride and lipid as well as microscopic fixative, decalcification, chromatography reagents.
The product is warts agent and astringent in pharmaceutical field, mainly used as biochemical drug extractant for the extraction of many highly efficient drugs such as adenosine triphosphate, cytochrome C and placental polysaccharides.
In addition, trichloroacetic acid, together with alkaline phenol, can be used for salicylaldehyde compound synthesis by ReimerTiemann reaction. It can also react with monoolefine compounds for synthesizing chlorocarboxylic acid [CCl3 (CH2CH2) nCOOH]. | [Definition]
ChEBI: A monocarboxylic acid that is acetic acid in which all three methyl hydrogens are substituted by chlorine. | [Preparation]
Trichloroacetic acid(76-03-9) is produced on an industrial scale by chlorination of acetic acid or chloroacetic acid mother-liquors at 140 - 160 °C. If necessary, calcium hypochlorite is added as a chlorination accelerator. There are conflicting views concerning adding heavy metal salts as chlorination catalysts. Examples of catalysts that have been used are iron and copper compounds, which are precipitated with sulfuric acid or phosphoric acid if decomposition of the reaction mixture occurs; 2% phosphoric acid; and catalysts and UV light. Trichloroacetic acid has also been produced without catalysts. The crude product, containing about 95% trichloroacetic acid, is best isolated by crystallizing the melt, removing the mother-liquor with most of its impurities, and increasing the purity by centrifugation or recrystallization.
| [Reactions]
Trichloroacetic acid is a strong organic acid with a dissociation constant K = 3 × 10-2. It has lively chemical properties. Its sodium salt is easily subject to decarboxylation into chloroform. It will be reduced to alcohol upon coming across LiAlH4. It can have halogen replacement reaction with KBr:
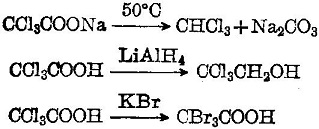 | [Biochem/physiol Actions]
TCA might act as a carcinogen, targeting liver. It might be used as an acid decalcifying agent. TCA helps in nuclear staining. | [Carcinogenicity]
TCA was not mutagenic in bacterial
assays.The IARC has determined that there is
limited evidence for the carcinogenicity of
TCA in experimental animals and that it is not
classifiable as to its carcinogenicity to humans. Neutralized TCA was not clastogenic
in human lymphocytes in vitro or in the mouse
bone marrow micronucleus test. | [Purification Methods]
Purify the acid by fractional crystallisation from its melt, then crystallise it repeatedly from dry *benzene and store it over conc H2SO4 in a vacuum desiccator. It can also be crystallised from CHCl3 or cyclohexane, and dried over P2O5 or Mg(ClO4)2 in a vacuum desiccator. Trichloroacetic acid can be fractionally distilled under reduced pressure from MgSO4. Layne, Jaffé and Zimmer [J Am Chem Soc 85 435 1963] dried trichloroacetic acid in *benzene by distilling off the *benzene-water azeotrope, then crystallised the acid from the remaining *benzene solution. Manipulations should be carried out under N2. [Toxic vapours, use a well ventilated fume cupboard.] [Beilstein 2 IV 508.] | [References]
https://pubchem.ncbi.nlm.nih.gov/compound/trichloroacetic_acid#section=Top
https://en.wikipedia.org/wiki/Trichloroacetic_acid |
| Questions and Answers (Q&A) | Back Directory | [Description]
Trichloroacetic acid (76-03-9) is a kind of acetate analogue with the three hydrogen atoms of the methyl group in acetate being replaced by chlorine atoms. It is a strong acid which can be used in protein precipitation in clinical chemistry and biochemistry as well as a caustic for warts removal. It can be synthesize through the reaction between chlorine and acetate in the presence of a suitable catalyst, or alternatively by the oxidation of trichloroacetaldehyde. In addition to protein precipitation, it can also be used for RNA and DNA precipitation. Its sodium salt can also be used as a herbicide.
|
|
|