| Identification | More | [Name]
ACETALDEHYDE AMMONIA | [CAS]
75-39-8 | [Synonyms]
1-AMINOETHANOL
ACETALDEHYDE AMMONIA
ACETALDEHYDE-AMMONIA TRIMER
ALDEHYDE AMMONIA
Acetaldehyde, amine salt
Aldamine
alpha-Aminoethyl alcohol
-Aminoethylalcohol
Ethanol, 1-amino-
Velsan
ACETALDEHYDE-AMMONIA TRIMER 98%
ACETALDEHYDE-AMMONIA TRIMER 3-HYDRATE
N-ethylhydroxyamine | [EINECS(EC#)]
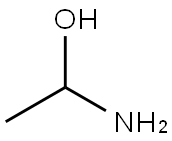
200-868-2 | [Molecular Formula]
C6H15N3 | [MDL Number]
MFCD00008058 | [Molecular Weight]
129.2 | [MOL File]
75-39-8.mol |
| Chemical Properties | Back Directory | [Description]
Acetaldehyde ammonia is a white crystalline solid and very soluble in water. It is used
to make other chemicals and vulcanise rubber. On long exposure to air, acetaldehyde
ammonia oxidises, hardens, and turns yellow or brown in colour and reacts exothermically
with water to evolve gaseous ammonia. Acetaldehyde ammonia reacts with strong
oxidising agents and halogens, and when heated, it readily decomposes into acetaldehyde
and ammonia. It attacks copper, aluminium, zinc, and their alloys and reacts with mercury
and silver oxides to form shock-sensitive compounds. | [Melting point ]
95-97°C (dec.) | [Boiling point ]
109-111°C | [density ]
0.9610 (estimate) | [refractive index ]
1.4521 (estimate) | [pka]
14.09±0.20(Predicted) | [color ]
White, crystalline solid | [CAS DataBase Reference]
75-39-8(CAS DataBase Reference) | [EPA Substance Registry System]
1-Aminoethanol (75-39-8) |
| Safety Data | Back Directory | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
1841 | [HazardClass ]
9 | [PackingGroup ]
III | [Safety Profile]
It readily decomposes into acetaldehyde and ammonia when heated, causing the hazards of these substances. Moderate fire and explosion hazard when exposed to heat or flame. Can react with oxidizing materials. When heated to decomposition it emits toxic fumes of NH3 and NOx |
| Hazard Information | Back Directory | [General Description]
A white crystalline solid. Melting point 97°C. Boiling point 110°C (with some decomposition). An addition product between acetaldehyde and ammonia. Presents moderate fire and explosion hazard when exposed to heat or flame. Moderately toxic by ingestion and inhalation and a strong irritant. Used to make other chemicals, vulcanize rubber. | [Reactivity Profile]
ACETALDEHYDE AMMONIA(75-39-8) reacts with strong oxidizing agents and halogens. Attacks copper, aluminum, zinc and their alloys. Reacts with mercury and silver oxides to form shock-sensitive compounds [Handling Chemicals Safely 1980. p. 139]. Readily decomposes into acetaldehyde and ammonia when heated, causing the hazards of those substances [Lewis]. | [Air & Water Reactions]
Resinifies (oxidizes, hardens and turns yellow or brown) on long exposure to air. Very soluble in water [Hawley]. Reacts exothermically with water to evolve gaseous ammonia. | [Health Hazard]
Inhalation of material may be harmful. Contact may cause burns to skin and eyes. Inhalation of Asbestos dust may have a damaging effect on the lungs. Fire may produce irritating, corrosive and/or toxic gases. Some liquids produce vapors that may cause dizziness or suffocation. Runoff from fire control may cause pollution. | [Fire Hazard]
Some may burn but none ignite readily. Containers may explode when heated. Some may be transported hot. | [Uses]
For preparing pure acetaldehyde; in organic syntheses. |
|
|